Booster for neutrophil granulocytes
Acetate supports immune cells to fight against sepsis
Blood poisoning is the most dangerous complication of bacterial infections and often leads to death. Researchers at the Interfaculty Institute of Microbiology and Infection Medicine at the University of Tübingen have now identified acetate as a potent agent for stimulating innate immune system cells, supporting their ability to destroy bacteria.
If the immune system fails to control a local infection, pathogens can spread throughout the body via the bloodstream and cause sepsis (blood poisoning). When this happens, a general inflammation of the organism occurs and, in the worst case, septic shock, which in the majority of cases leads to death due to multiple organ failure. Every year, at least 75,000 people in Germany die from sepsis, significantly more than from breast, prostate and colon cancer combined. The cause of sepsis is a misdirected immune response: the inflammatory reactions deployed to fight the invaders become out of control and damage the body's own tissues and organs.
Neutrophil granulocytes fight invaders
Pathogens are primarily eliminated by the immune cells of the innate immune defense circulating in blood vessels and tissues. Neutrophil granulocytes (neutrophils) in particular, the most common white blood cells, are able to identify and destroy invaders. Special receptors on the surface of these neutrophils help bind to pathogen-specific structures, activating the cells that can eliminate (phagocytosis) or kill the microorganisms by releasing toxic substances. In addition, the neutrophils release messenger substances that attract further immune cells. Moreover, the substances released by the neutrophils and newly attracted immune cells make the blood vessels wider and more permeable, thus causing the infected region to become inflamed and enabling optimal control of the pathogen.
 PD Dr. Dorothee Kretschmer and Dr. Katja Schlatterer discovered the stimulatory effect of acetate on neutrophil granulocytes. © University of Tübingen
PD Dr. Dorothee Kretschmer and Dr. Katja Schlatterer discovered the stimulatory effect of acetate on neutrophil granulocytes. © University of TübingenNeutrophils have a large number of different receptors embedded in their cell membrane. These receptors recognise microbe-associated molecular patterns (MAMP), which can be proteins, carbohydrates or lipids that are located in or on the pathogen or are secreted by it. The receptors’ recognition patterns are stored in the cells’ genetic information and – unlike the receptors of antibodies of the acquired immune response – cannot be adapted in the course of an infection.
PD Dr. Dorothee Kretschmer’s research group at the Interfaculty Institute of Microbiology and Infection Medicine at the University of Tübingen (IMIT) has been studying the formyl peptide receptor (FPR) class for more than ten years. These receptors are mainly found on neutrophils and recognise short peptides with a formyl residue. These formylated peptides are mainly released by bacteria because newly synthesised bacterial proteins begin with the amino acid formylmethionine. FPRs belong to the family of G-protein-coupled receptors that, when activated, trigger a signalling cascade inside the cell mediated by GTP-binding proteins. The neutrophils then migrate towards the site of infection (chemotaxis) and phagocytosis activity is enhanced.
Acetate triggers alarm state in neutrophils
As part of the "Control of Microorganisms to Fight Infections (CMFI)" cluster of excellence, the researchers searched for other bacterial molecules that trigger similar effects. The goal of the alliance of Tübingen research groups from the university and the Max Planck Institute for Developmental Biology is to develop new forms of therapy to displace harmful bacteria from the human microbiome.
In their analyses, the researchers discovered short-chain fatty acids (SCFA) such as acetate and propionate, which are recognised by GPR43. This is a G protein-coupled receptor found on cells of the intestinal epithelium, as well as on neutrophils. "For a long time, it was very difficult to determine the function of GPR43, because we were unable to observe any activating mechanisms," reports Dr. Katja Schlatterer, first author of the recently published study.1) "Only the combination with the FPR project brought the breakthrough and led us to the priming mechanism." During priming, the cells are put on alert by acetate so that they can react more effectively to subsequent contact with other bacterial ligands. They then secrete larger amounts of messenger substances (e.g. interleukin-8) and toxic radicals and migrate more quickly to the site of infection. Stimulation by acetate also leads to the upregulation of other receptors important for bacterial recognition, thus increasing neutrophils’ capacity to undergo phagocytosis.
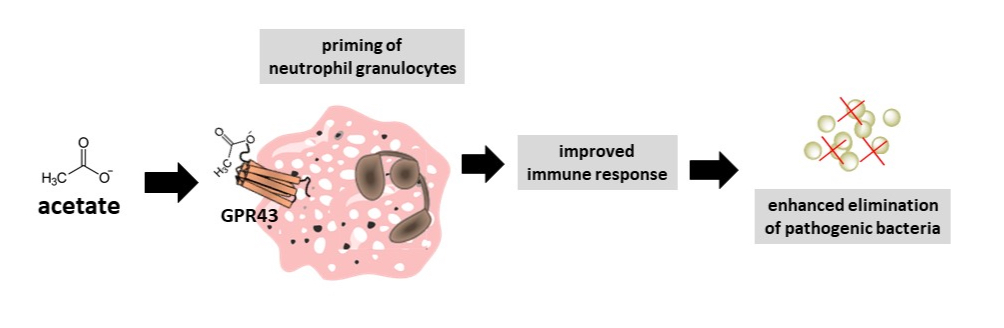 The acetate molecule binds to GPR43 on the surface of neutrophil granulocytes, putting them in a state of alert and thus increasing their immune activity. © University of Tübingen
The acetate molecule binds to GPR43 on the surface of neutrophil granulocytes, putting them in a state of alert and thus increasing their immune activity. © University of Tübingen
Therapeutic use of acetate conceivable
Studies in mice also confirmed the efficacy of acetate in combating systemic infections. If the animals were given acetate in the form of an injection or via drinking water before infection with Staphylococcus aureus, the main cause of bacterial sepsis, they showed significantly milder disease symptoms. However, acetate not only has a preventive effect, but also direct therapeutic benefits and was able to significantly improve the course of pre-existing sepsis. These results give justified reason to hope that acetate can also be used to treat bacterial sepsis in humans.
Acetate is non-toxic and is released in relatively large amounts when food is digested in the intestine. However, the amount in blood serum varies greatly between individuals, depending on diet and metabolic activity. "Acetate is already being used for clinical purposes as buffers in many infusion solutions," Kretschmer explains. " It is therefore worth considering whether a targeted boost with an acetate solution could be given when sepsis is diagnosed." This would be conceivable in combination with the administration of antibiotics. Although tolerability has already been proven, the use of acetate for this purpose still needs to be tested in clinical trials.
The importance of the GPR43-mediated signalling pathway for the body's immune defense is supported by the observation that sepsis patients with an increased number of GPR43 receptors on their blood cells have a better chance of survival. Priming by acetate therefore has great potential as an effective supplement for treating bacterial infections, particularly given the growth in antibiotic-resistant bacteria.