Biosensor for whole blood and exhaled breath analysis
Antibiotic detection from whole blood or exhaled breath possible
Incorrectly dosed antibiotics are not only dangerous for patients, but also often the cause of resistant strains of bacteria. Researchers at the University of Freiburg have developed a biosensor to determine the effective amount and thus enable personalised therapy. The biosensor works by rapidly determining small amounts of the substances directly from whole blood or exhaled breath.
Antibiotics are the drug of choice for treating bacterial infections. They prevent pathogens from multiplying or even kill them. Naturally occurring antibiotics are formed by moulds as well as bacteria. "Life prevents life," Louis Pasteur noted as early as 1877, describing how one type of bacteria stops another from growing. Shortly afterwards (1893), the Italian physician Bartolomeo Gosio succeeded in isolating a substance from a fungus of the genus Penicillium that was able to stop anthrax pathogens reproducing. However, this research attracted little international attention, so today Alexander Fleming is officially considered the discoverer of penicillin. Fleming discovered penicillin by accident while he was investigating staphylococcus, and a Penicillium mould was accidentally introduced into the culture plates, thus preventing the growth of the bacteria. He was awarded the Nobel Prize in Physiology and Medicine in 1945 for his subsequent work in understanding the action of penicillin and for the development of therapeutic agents against a large number of bacterial diseases.
Penicillins act primarily against bacteria whose cell membrane is covered by a thick murein (peptidoglycan) layer, a macromolecule made of glycan strands (consisting of two alternating amino sugars) cross-linked by small peptides. Gram-positive pathogens – which appear blue after Gram staining, a test to classify bacteria into two broad categories according to their cell wall type – include Staphylococcus aureus (a common cause of sepsis) and pneumococci (a cause of pneumonia), as well as anthrax and diphtheria pathogens. The antibiotics inhibit a group of bacterial enzymes essential for murein synthesis called penicillin-binding proteins (PBPs). As a result, the newly synthesised cell wall becomes unstable, and the bacteria eventually burst as water rushes into the cell. Penicillins therefore only kill cells that are growing and dividing.
Uncontrolled use of antibiotics generates resistance
 Dr. Can Dincer, together with his colleagues H. Ceren Ates and Regina T. Glatz, developed a biosensor that can detect the smallest amounts of antibiotics in various body fluids. © Patrick Seeger/University of Freiburg
Dr. Can Dincer, together with his colleagues H. Ceren Ates and Regina T. Glatz, developed a biosensor that can detect the smallest amounts of antibiotics in various body fluids. © Patrick Seeger/University of FreiburgAs early as World War 2, penicillin derivatives were used on the battlefields and saved the lives of many soldiers. They were long considered miracle cures and were used without major restrictions, not just in humans, but later also in factory farming. This widespread use in particular, but also dosages that were too low or intake periods that were too short, enabled the bacteria to develop resistances to these antibiotics. As a result, several thousand people die each year in Germany due to multiresistant pathogens. "We need personalised antibiotic therapies that define the start and end of intake, but most of all precisely define the dose. The effective amount in the blood must be high enough to prevent resistance, but must not lead to organ damage," explains Dr. Can Dincer from the Freiburg Centre for Interactive Materials and Bioinspired Technologies at the University of Freiburg (FIT) and head of the Disposable Microsystems working group at the Department of Microsystems Engineering (IMTEK).
Biosensor for rapidly detecting penicillin concentration
For this purpose, he developed a special biosensor that can detect the smallest amounts of penicillins. The development was undertaken within the framework of a DFG-funded project in which Dr. Dincer worked with the Synthetic Biology research group led by Prof. Dr. Wilfried Weber (CIBSS - Centre for Integrative Biological Signalling Studies at the University of Freiburg) and the Clinical Respiratory Physiology research group led by Prof. Dr. Stefan Schumann (Department of Anaesthesiology and Intensive Care Medicine at the Freiburg University Medical Centre).1) The immobilisation zone of the sensor is coated with a synthetically produced PBP that recognises a ß-lactam ring, a central molecular structure of penicillins. The antibiotic contained in the sample competes with a ß-lactam molecule conjugated to the enzyme glucose oxidase for binding to PBP onthe sensor. This ß-lactam molecule is conjugated to the enzyme glucose oxidase. The addition of glucose leads to its conversion into hydrogen peroxide (H2O2) by the glucose oxidase enzyme. In the electrochemical cell of the sensor, which is located downstream of the sensor’s immobilisation zone, the amount of H2O2 produced is detected amperometrically as a current flow. The decrease in current serves as a measure of the amount of penicillin in the analyte. Comparative measurements using the complex HPLC (high performance liquid chromatography) method at Freiburg University Medical Centre confirmed the accuracy of this new technique. It can detect antibiotic concentrations in the ng/ml range in less than 90 minutes. This makes it up to 500 times more sensitive than conventional detection methods.
Determination from whole blood or exhaled breath
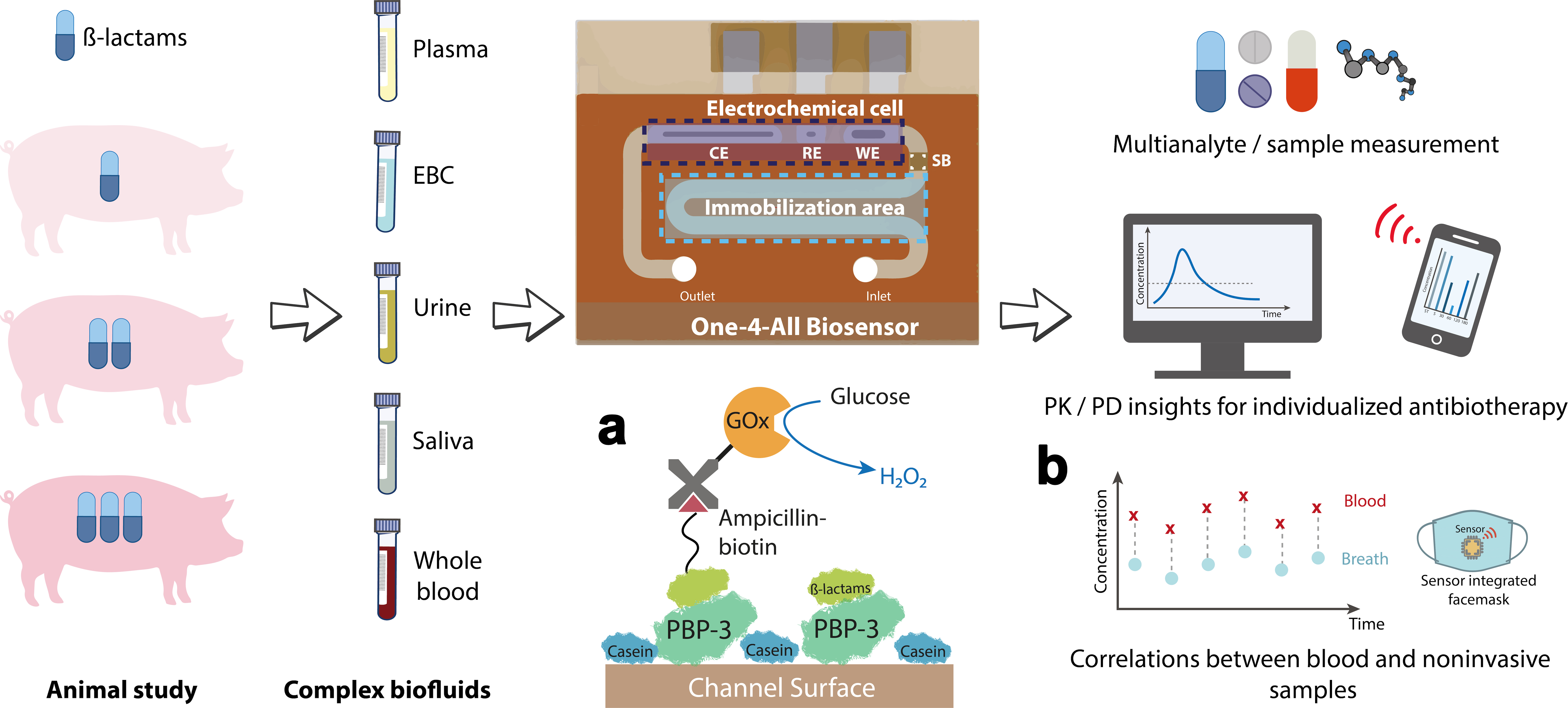 Analyses using the novel biosensor were performed on various body fluids from pigs that had received different amounts of antibiotics. a) The sensor surface is coated with a penicillin-binding protein (PBP) that can bind either a ß-lactam antibiotic or ampicillin linked to glucose oxidase (GOx). GOx generates hydrogen peroxide (H2O2) in the presence of glucose, the amount of which is measurable as a current flow. b) The antibiotic concentration measured in exhaled breath condensate correlates directly with the antibiotic level in the blood, which makes a face mask with an integrated sensor feasible in the long term. Source: C. Dincer, https://doi.org/10.1002/adma.202104555, CC-BY 4.0, translated by C. Dincer
Analyses using the novel biosensor were performed on various body fluids from pigs that had received different amounts of antibiotics. a) The sensor surface is coated with a penicillin-binding protein (PBP) that can bind either a ß-lactam antibiotic or ampicillin linked to glucose oxidase (GOx). GOx generates hydrogen peroxide (H2O2) in the presence of glucose, the amount of which is measurable as a current flow. b) The antibiotic concentration measured in exhaled breath condensate correlates directly with the antibiotic level in the blood, which makes a face mask with an integrated sensor feasible in the long term. Source: C. Dincer, https://doi.org/10.1002/adma.202104555, CC-BY 4.0, translated by C. Dincer"A real highlight is the ability to analyse whole blood, which is comparable to monitoring blood glucose concentrations with the test strips used by diabetics," says Dincer, describing a special feature of the biosensor. "This allows for uncomplicated application." But the sensor offers even more: it’s not just blood (either in the form of plasma or directly as whole blood) that is suitable as a starting material but also non-invasive samples such as saliva, urine or even exhaled breath. The latter in particular opens up new options for the timely monitoring of effective antibiotic levels in the body. Until now, this has only been possible by means of plasma analysis. Urine samples are not suitable for acute monitoring because it takes too long for the kidneys to filter the blood and the substances to be excreted. There is also a certain delay before substances reach the saliva. "The lungs, on the other hand, have a strong blood supply, so a rapid exchange of substances takes place. With the help of the sensitive biosensor, we have now shown that there is an exact correlation between our measured values from the exhaled breath condensate and those in the plasma," reports the microsystems engineer.
The investigations were carried out on pigs that had received different doses of antibiotics. During thirty minutes, the exhaled breath was collected in a mask from anaesthetised animals, before being condensed and then analysed. However, this method is too cumbersome for routine use. In another project, Dincer's group is therefore trying to develop a mask with integrated exhaled breath sensors.2) In addition, the influence of lung function disorders, such as asthma, on the result measured will be investigated in the future.
Wide use of the biosensor is possible
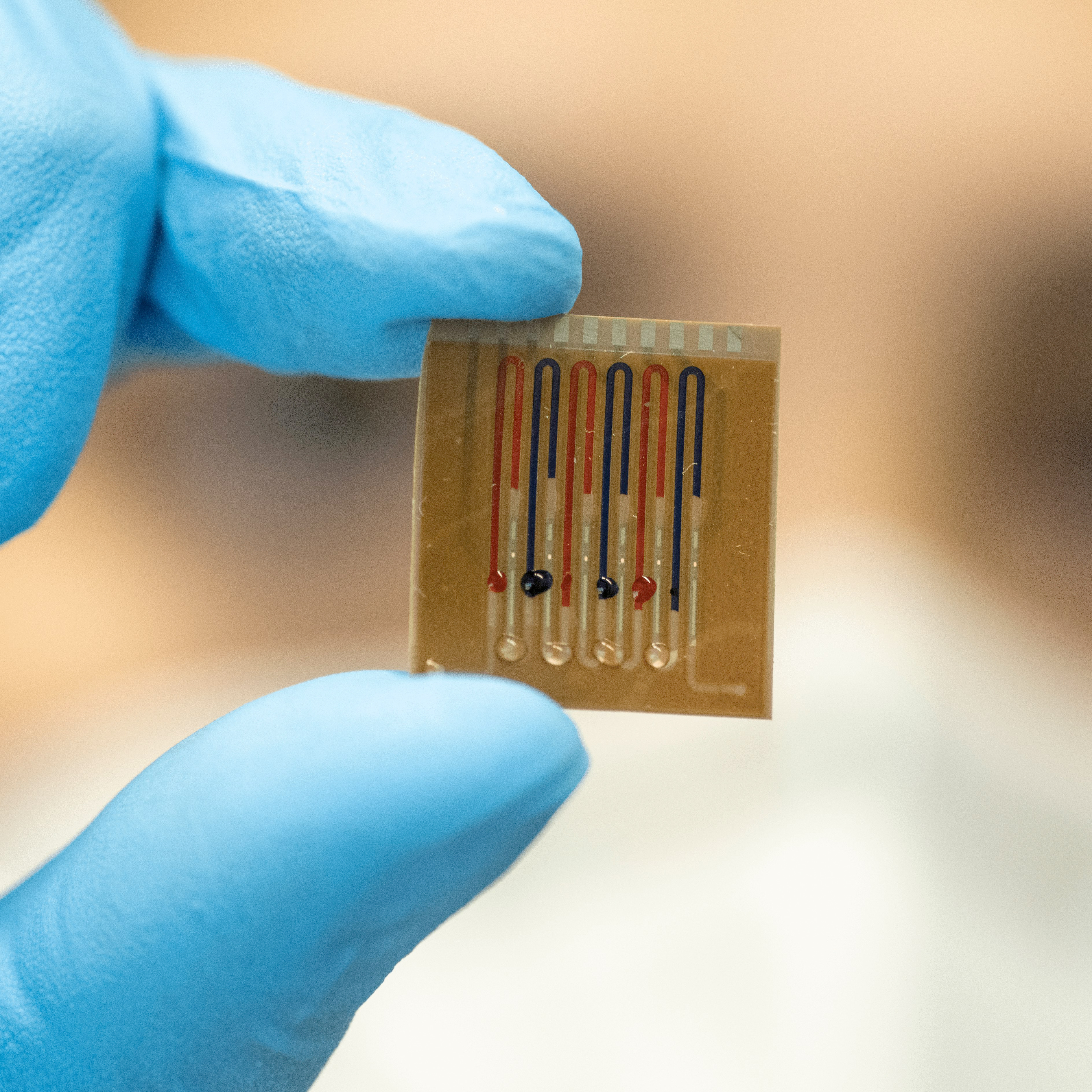 The multiplex chip can be used to examine different samples and substances. © Patrick Seeger/University of Freiburg
The multiplex chip can be used to examine different samples and substances. © Patrick Seeger/University of FreiburgSince the PBP immobilised on the biosensor recognises the general ß-lactam backbone, all members of this antibiotic class (including cephalosporins) can be detected, which opens up a wide range of applications. Furthermore, the researchers have already constructed a multiplex chip that contains four parallel channels and can be used to analyse either different samples or different substances. "Our ultimate goal is to take a sample and be able to determine in parallel other parameters, such as inflammation or kidney markers, in addition to the amount of antibiotics," Dincer says about his future plans. "This is particularly important in a clinical setting for sepsis or post-transplant patients."
During the interview, the scientist emphasised that the development of the innovative biosensor was mainly made possible thanks to good cooperation between the university institutes and hospitals in Freiburg that work together on joint DFG projects.
The maxim 'as much as necessary, as little as possible' is more important than ever when dealing with antibiotics. The work of the Freiburg researchers lays important foundations for future, well-dosed individual therapies.