Atriva Therapeutics
COVID-19 pioneer drug in Phase II clinical trial – with double the power
The effective treatment of people with severe COVID-19 is a major goal during the corona pandemic. ATR-002, an oral small molecule that targets RNA viruses such as influenza virus and SARS-CoV-2, has a dual effect: it impairs viral propagation and also has an immunomodulatory effect. And what’s more, due to its unique cellular mechanism of action, the efficacy of Atrivia Therapeutics’ drug candidate is not reduced by virus mutations and resistances.
Most of the drugs approved against RNA viruses to date target viral components. Atriva Therapeutics, a biopharmaceutical start-up company based in Tübingen, commenced a clinical Phase II clinical trial in July 2020 with a drug candidate that has a completely different mode of action. Instead of targeting the coronavirus itself, the company’s drug candidate ATR-002 targets human host cells. The advantage of targeting human host cells instead of viral proteins is that the efficacy of ATR-002 is not reduced once specific mutations appear in the virus. The risk of resistance developing is thus considerably lower. ATR-002 blocks a signalling pathway in infected human cells, such as lung cells, that is essential for the replication of RNA viruses. This prevents the formation of functional new viral particles, and hence the further spread of the virus.
Seed funding speeds research towards Phase II clinical testing
 Prof. Dr. Oliver Planz is co-founder and Chief Scientfic Officer der Atriva Therapeutics GmbH. © Atriva Therapeutics GmbH
Prof. Dr. Oliver Planz is co-founder and Chief Scientfic Officer der Atriva Therapeutics GmbH. © Atriva Therapeutics GmbHHow can a drug like this be developed so quickly and how has it already been in clinical Phase II testing since July 2020? Atriva uses compounds that the company has already tested in clinical trials. The university spin-off led by Prof. Dr. Oliver Planz, University of Tübingen, Prof. Dr. Stephan Ludwig, University of Münster, and Prof. Dr. Stephan Pleschka, University of Gießen, has been working on the development of pharmaceutically active ingredients against influenza viruses since 2015. "Already in February 2020, we believed that the spread of SARS-CoV-2 would assume pandemic proportions. We therefore decided to use our multi-year preliminary work on our influenza drug candidate, which was already undergoing clinical Phase I testing, as the basis for COVID-19 drug development1,2," says co-founder and CEO Dr. Rainer Lichtenberger, explaining the company’s lead in COVID-19 drug development. This is almost a quantum leap considering how much time drug development otherwise takes.
"As catastrophic as the current corona pandemic is, it nevertheless shows how important it is to support new ideas for combating viral respiratory diseases at an early stage. Especially alternative therapeutic approaches that are not the focus of the pharmaceutical industry and venture capital firms." Dr. Lichtenberger knows the obstacles - and the omissions – very well. "Over the years, it has become increasingly difficult to obtain follow-up funding. This has significantly influenced the speed of drug development." At the same time, the danger posed by coronaviruses and the simultaneous lack of medication has been well known since the SARS epidemic in 2003. Threats from virus infections must be taken seriously, so action can be taken against similar catastrophes in the future. "We are very pleased that the Ministry of Research intends to make healthcare projects, in addition to digitalisation and climate technology projects, eligible for funding through the planned investment fund for future technologies."
Seed investors, including the High-Tech Gründerfonds (HTGF) with investors such as the BMWi (Baden-Württemberg Ministry of Economic Affairs and Energy) and the KfW (German state-owned development bank), are important for Atriva. Together with other life sciences investors, they enable Atriva to advance its research platforms for therapeutics against viral influenza and other life-threatening respiratory diseases.
Good starting position for the ATR-002 drug candidate
The oral small molecule ATR-002, which was specifically developed to treat diseases caused by RNA viruses such as influenza viruses or coronaviruses, including SARS-CoV-2, is Atriva’s lead product. "In preclinical studies, we were able to demonstrate that ATR-002 was highly effective against the influenza virus. A dose escalation study demonstrated the molecule’s excellent safety and tolerability," said Prof. Dr. Oliver Planz, co-founder and CSO of Atriva.
A substance can only be tested on humans if it has passed all the required preclinical tests and been shown to be safe in animal experiments. Atriva has already achieved all this and can therefore use its clinical stage drug candidate for assessing its efficacy in treating other infections. "We have already demonstrated strong efficacy against RSV (respiratory syncytial virus) and hantavirus." Since July 2020, the company has turned to the new coronavirus: in a multinational, double-blind clinical Phase II study, the company is assessing the efficacy of ATR-002 in 200 patients with moderately severe COVID-19 in comparison with a placebo. "Compared with influenza that causes over half a million deaths globally a year, the coronavirus pandemic has already exceeded this number," says Dr. Lichtenberger highlighting the need for an effective medicine to be quickly available. This need is also underlined by the fact that further pandemics can occur; only recently scientists warned about a new form of swine flu.3
Chain reaction stopped for a short period of time
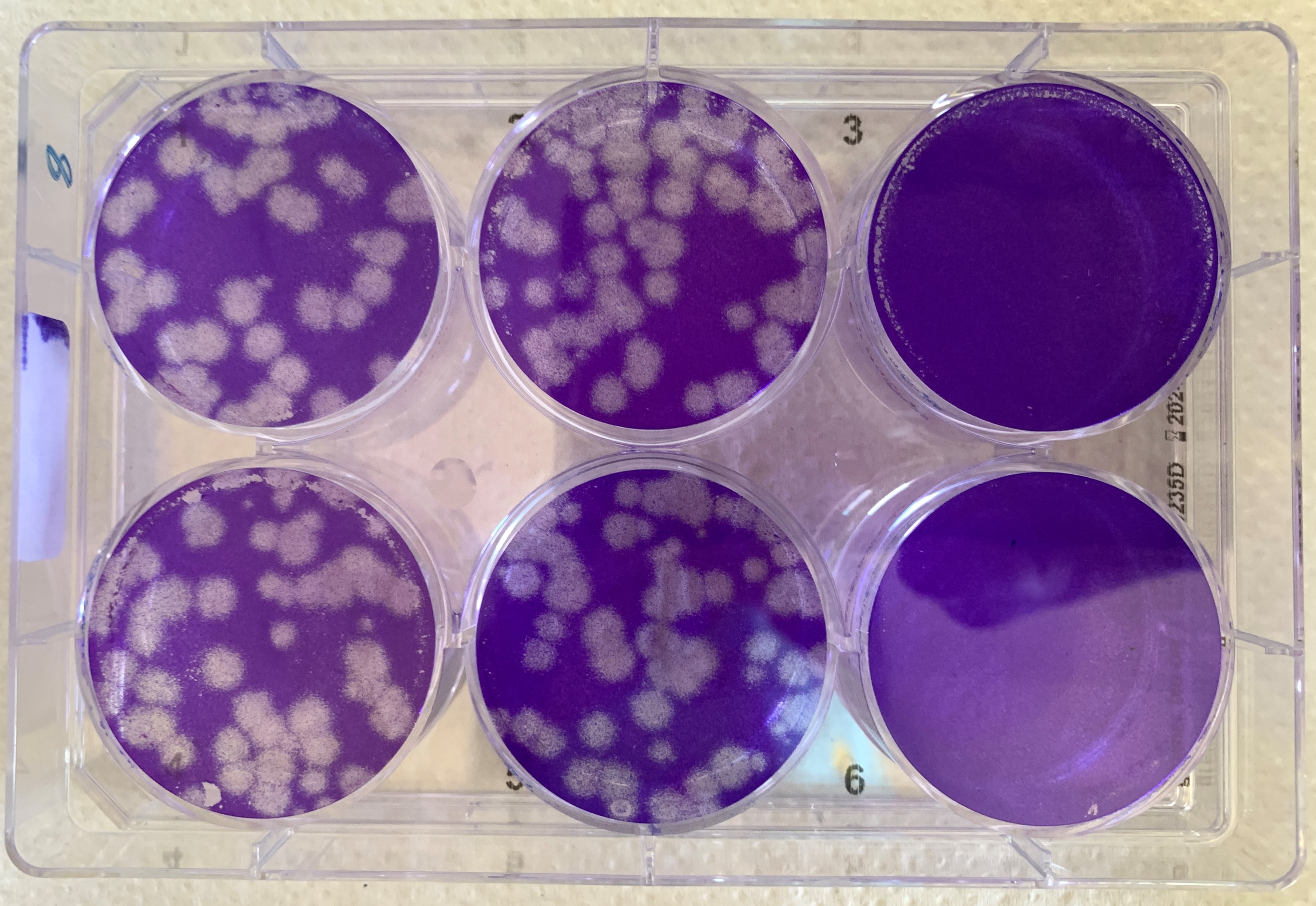 SARS-CoV-2 was added to all culture dishes with a dense lawn of blue stained cells. The dishes in the left-hand and central row show characteristic white holes in the cell lawn. That is where the virus has destroyed the cells. The dishes in the right-hand row, to which ATR-002 was also added, do not show white areas. This is a sign that the cells have not been destroyed. © Helen Hoffmann, Atriva Therapeutics GmbH
SARS-CoV-2 was added to all culture dishes with a dense lawn of blue stained cells. The dishes in the left-hand and central row show characteristic white holes in the cell lawn. That is where the virus has destroyed the cells. The dishes in the right-hand row, to which ATR-002 was also added, do not show white areas. This is a sign that the cells have not been destroyed. © Helen Hoffmann, Atriva Therapeutics GmbHWhere does Atriva’s candidate exert its action? In June, scientists led by Atriva’s co-founder Prof. Dr. Stephan Ludwig achieved major success in their research on influenza virus infection: they were able to demonstrate the complete mechanism of a signalling pathway that influenza viruses need for replication. This finding was published in the renowned scientific journal PNAS4. They were also able to precisely pinpoint the target of attack of their drug candidate: "ATR-002 inhibits the enzyme MEK, which is an essential component of the so-called Raf/MEK/ERK signalling pathway", said Prof. Dr. Planz.
The cellular Raf/MEK/ERK signalling pathway consists of a chain of enzymes that communicate a signal from a cell surface receptor to DNA in the cell nucleus. The enzymes (kinases) activate themselves in the cascade one after the other by attaching phosphate groups. This process is called phosphorylation. MEK is one such enzyme. When inhibited by ATR-002, RNA viruses can no longer use the cascade for themselves. As a result, virus replication or export from the cell is blocked and the viral load in the infection process is reduced. Atriva's drug candidate has already completed Phase I testing and can therefore be tested directly in Phase II clinical trials for its effect on any RNA virus that depends on the Raf/MEK/ERK signalling pathway for replication.
"With ATR-002, the signalling pathway mechanism remains intact. It is blocked just for a brief period of time, so that the virus cannot use it for its purposes," explains Prof. Dr. Planz. "We believe that the short treatment time does not lead to any adverse effects."
Beyond that, ATR-002 has the potential to avoid a virus-induced excessive cytokine response. MEK inhibition suppresses the production of cytokines, such as certain tumour necrosis factors and interleukins, and thus minimizes inflammatory reactions in the lung.
"Ultimately, our results support the strategy of advancing ATR-002 as a treatment for many viral respiratory infections that depend on the Raf/MEK/ERK signalling pathway," said Prof. Dr. Planz. It is therefore planned to launch a Phase II clinical trial for influenza next winter.
The fact that ATR-002, which can be taken easily orally and makes the development of viral drug resistance far less likely, has a dual advantage over existing antivirals, makes the molecule a very promising drug - whether it will be used for the treatment of influenza or COVID-19. It will be interesting to see the results of the ongoing Phase II clinical trial.