Viral zoonoses
Development of inhibitors that help stop viral zoonoses
Emerging viral infections such as COVID-19 or Zika disease pose an increasing threat to humans. At the Institute of Pharmacy and Molecular Biotechnology (IPMB) at Heidelberg University, Prof. Dr. Christian Klein's research group is developing inhibitors against already known viruses in the hope that these can also be used against new virus variants.
Close contact between humans and animals favours mutual infection with pathogens. This can have fatal consequences, as the example of COVID-19 shows. Zoonoses – diseases that are transmissible from animals to humans – are caused by viruses, bacteria, parasites, prions or fungi. Transmission takes place either directly through contact with the infected animal or contaminated food (for example, in the case of Salmonella infections) or via an intermediate host such as ticks (Lyme disease) or mosquitoes (malaria). Due to the high mobility of humans and their intrusion into previously uninhabited habitats, new, dangerous zoonotic diseases such as Ebola or Zika have increasingly appeared in recent years.
Looking for active substances against zoonotic viruses
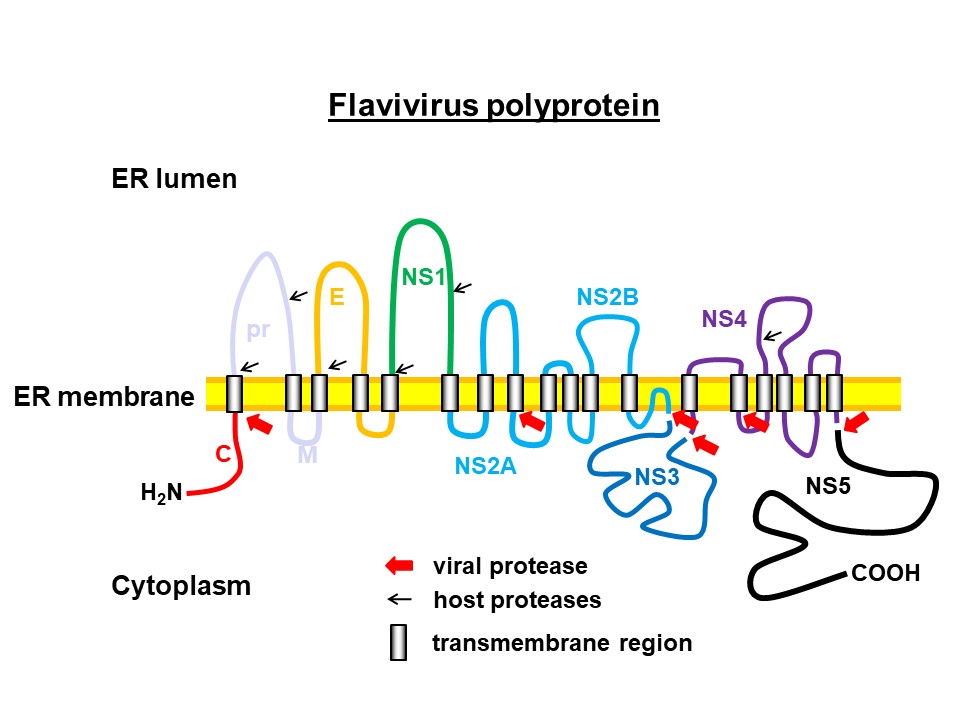 Based on the (+)-RNA template, the viral protein is synthesised at the rough endoplasmic reticulum (ER) and incorporated into the ER membrane. Viral as well as host proteases release the different viral proteins by cleaving the protein chain. © C. Klein
Based on the (+)-RNA template, the viral protein is synthesised at the rough endoplasmic reticulum (ER) and incorporated into the ER membrane. Viral as well as host proteases release the different viral proteins by cleaving the protein chain. © C. KleinThe Pharmaceutical and Medicinal Chemistry research group led by Prof. Dr. Christian Klein at the Institute of Pharmacy and Molecular Biotechnology (IPMB) at Heidelberg University has been researching active substances against viral zoonotic diseases for more than twelve years. The researchers' focus is on diseases caused by the genus Flavivirus. These include early summer meningoencephalitis (FSME), which is spread by ticks in Germany, but also Zika disease, dengue and West Nile fever, which are more common in the tropics. The latter are transmitted by mosquitoes and often have life-threatening courses.
The genetic information of the infectious flavivirus particles (virions), which are 40 - 60 nm in diameter, consists of a (+)-RNA single strand that is packaged in a protein capsid and surrounded by a membrane envelope. In addition to endothelial cells at the injection site, the virions also infect immune cells such as macrophages, monocytes and dendritic cells, among others, through which they are distributed throughout the body. In the host cells, the viral RNA serves as a direct template for the synthesis of a long polyprotein, i.e. a chain of interlinked, different viral proteins. The individual proteins are released by cleavage of the chain by proteases and can then fulfill their function to replicate the pathogen. This essential process is mainly carried out by the virus's own enzymes, which are also part of the polyprotein.
Protease inhibitors prevent multiplication of the pathogen
Since pathogen replication is not possible without cleavage of the long protein chains, viral proteases are an important target for therapeutics. Substances that block the active site of the enzymes (protease inhibitors) are already being used for the treatment of some viral infections. "The fact that HIV infection has evolved from a fatal disease to a controllable chronic disease with high life expectancy is largely thanks to protease inhibitors," Klein explains. However, these inhibitors are not universally applicable, but have to be developed specifically for each viral genus.
The search for effective inhibitors normally begins with a biochemical test system (assay) in which chemical substances are tested for their effectiveness on the isolated viral protease. The molecules identified are subsequently characterised in more detail in biological models, for example, culture cells infected with viruses, before their effectiveness is then investigated in living organisms. "Unfortunately, over the years we have had to learn that biochemical assays do not represent biological reality. Substances that work on the isolated target protein often do not work when the protease is in its biological microenvironment in the cell," says the pharmacist, describing the difficulties encountered. "That's where the enzyme localises to a specific area of the cell or interacts with other proteins, so it's not as accessible to the inhibitors or is in a slightly different 3D shape."
Reporter gene assay with huge predictive power
 In the search for effective protease inhibitors, Prof. Dr. Christian Klein and his team from the IPMB at the University of Heidelberg developed a cell-based reporter gene assay. © C. Klein
In the search for effective protease inhibitors, Prof. Dr. Christian Klein and his team from the IPMB at the University of Heidelberg developed a cell-based reporter gene assay. © C. KleinBased on many years of experience, the Heidelberg research group has now developed a cell-based test system for the primary characterisation of substances against the dengue virus, a so-called reporter gene assay. In this assay, instead of introducing fully functional, dangerous viruses into culture cells, genetic information for the viral protease is introduced instead. In addition, the cells contain a reporter protein (luciferase in this case) that is linked via a virus-specific cleavage site to a sequence that triggers the degradation of the protein. The viral protease cleaves the protein chain, preventing the degradation of the luciferase, whose activity is analysed after the disruption of the cells. Protease inhibitors reduce cleavage, luciferase becomes unstable, and its overall activity in cells decreases.
Since the effectiveness of inhibitors is examined by simply adding the substances to the culture medium, a reduced signal not only indicates effective inhibition of the protease, but also shows that the substance can penetrate the cell membrane and is sufficiently stable and non-toxic. "Our reporter gene assay has much better predictive power than conventional systems. Unsuitable substances are identified and rejected early on," Klein elaborates. In this way, the researchers have already successfully identified active substances against dengue infections and optimised them through chemical modifications. The substances are currently being tested in preclinical studies.
In August 2021, Klein received a 450,000 euro grant from the Volkswagen Foundation for his work on the transfer of the reporter gene assay to other flaviviruses and to coronaviruses. The grant was part of a project called ‘Viral Zoonoses – Innovative Approaches in Drug Development’. The grant application was approved on the basis of the extensive institutional knowledge and experience at the IPMB that lead to a high reproducibility of the experiments, as well as another important criterion, which was the existing close cooperation with Protinhi Therapeutics. The Dutch company specialises in protease inhibitors and has been investigating the substances found by the research group in the preclinical phase for several years, as well as developing new active substances that are biochemically analysed by the researchers.
Fundamental research prepares for new threats
"We mainly do basic research. This has enormous value because it creates preparedness for future situations," says Klein, explaining his lab's strategy. "In flaviviruses, for example, the recognition sequences of the proteases are relatively constant. If we develop inhibitory compounds now, there's a chance we'll already have inhibitors in the cupboard for the next new virus." For example, after the large 2015 outbreak of Zika virus infections in Latin America, the inhibitors developed against the dengue virus enabled rapid structural elucidation of the Zika virus protease.