Antibody molecules (immunoglobulins) are composed of two identical long (heavy) and two identical short (light) protein chains joined to form an upsilon-shaped molecule. Each of the heavy chains consists of four compactly folded regions (domains), whereas the light chains consist of only two. At the tip of each chain is a so-called variable region with unique properties determining the specificity of the antibody. The variable regions of a heavy chain and a light chain together form the specific recognition site of an antibody. The antibody uses this region (antigen-binding site) to attach to a suitable structure (antigen) on the pathogen. An antigen can be bound by different antibodies that recognise different areas (epitopes) within the antigen.
The so-called constant regions of an antibody are identical in all antibodies of the same class. Class M immunoglobulins (IgM), for example, are formed during the acute infection phase. IgGs are formed at a later time or after vaccination and are part of the immunological memory. After binding an antigen, other components of the immune system are activated via the constant part of the antibody so that the pathogen can be destroyed.
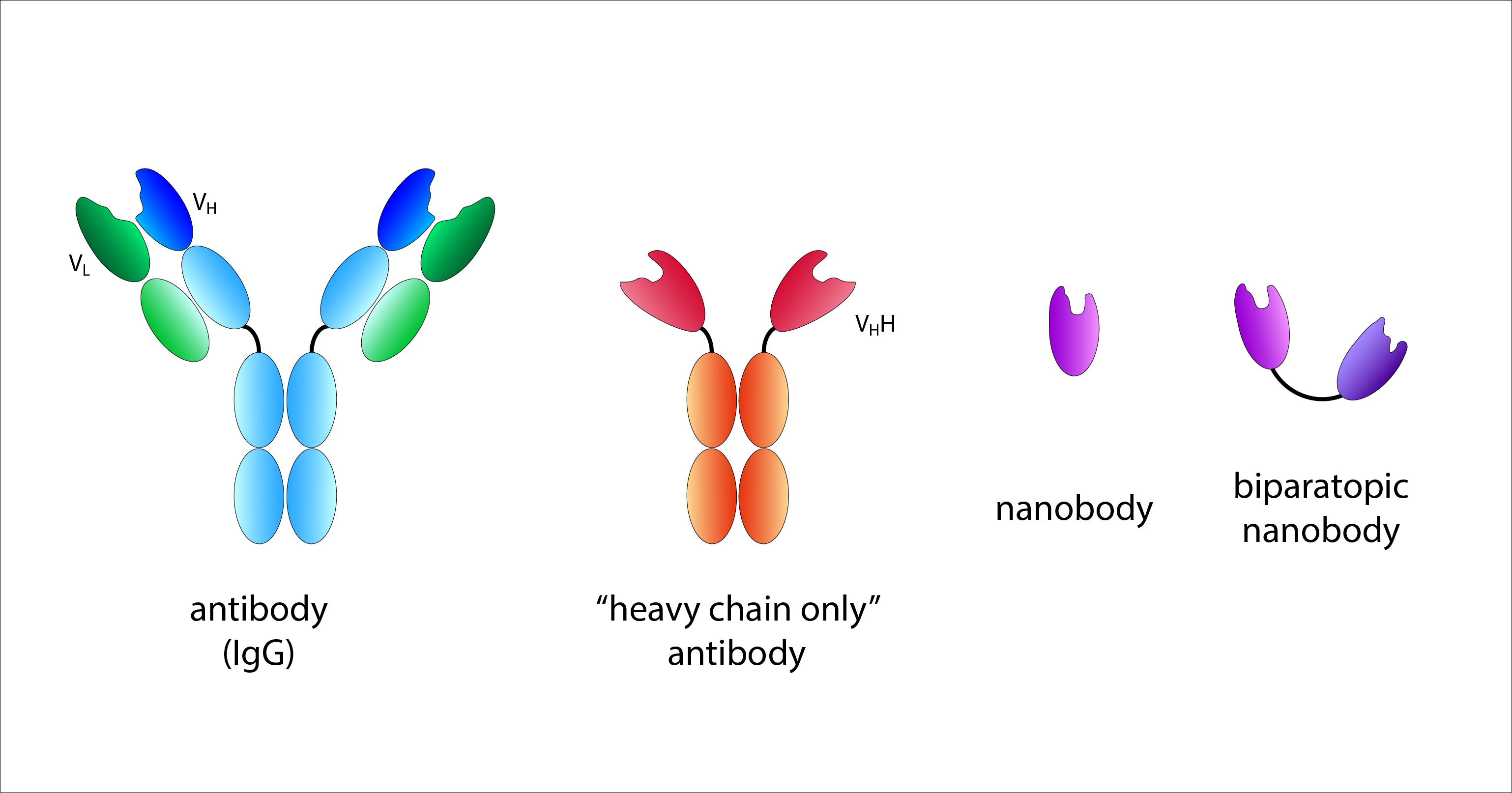 The antigen recognition site of a conventional antibody is formed jointly by the variable regions of the heavy (VH) and light (VL) chains. A nanobody corresponds to the variable region of a ‘heavy chain only’ antibody from camelids. Biparatopic nanobodies have two recognition domains with different specificities. © R. Menßen-Franz
The antigen recognition site of a conventional antibody is formed jointly by the variable regions of the heavy (VH) and light (VL) chains. A nanobody corresponds to the variable region of a ‘heavy chain only’ antibody from camelids. Biparatopic nanobodies have two recognition domains with different specificities. © R. Menßen-FranzAntibodies of different classes are produced by all higher vertebrates and are found in the blood (IgG), tissue fluid and body secretions (IgA). Interestingly, members of the Camelidae family (camelids) (e.g. camels, alpacas, llamas, dromedaries) also possess another form of immunoglobulins, so-called ‘heavy chain only’ antibodies. These consist exclusively of two identical heavy chains, so the complete antigen-binding site is formed by only one variable region. This special feature was first described in 1993 and has since been increasingly used in research and therapy.
After an animal has been immunised, B lymphocytes can be isolated from its blood in order to then create gene libraries containing only the information required to produce the variable regions of the ‘heavy chain only’ antibodies. These single-domain antibody fragments, so-called nanobodies, have very good antigen binding properties and a number of advantages compared with conventional antibodies. Nanobodies are significantly smaller and more stable and can be produced easily and cheaply. At the same time, they open up new possibilities in biomedical research.
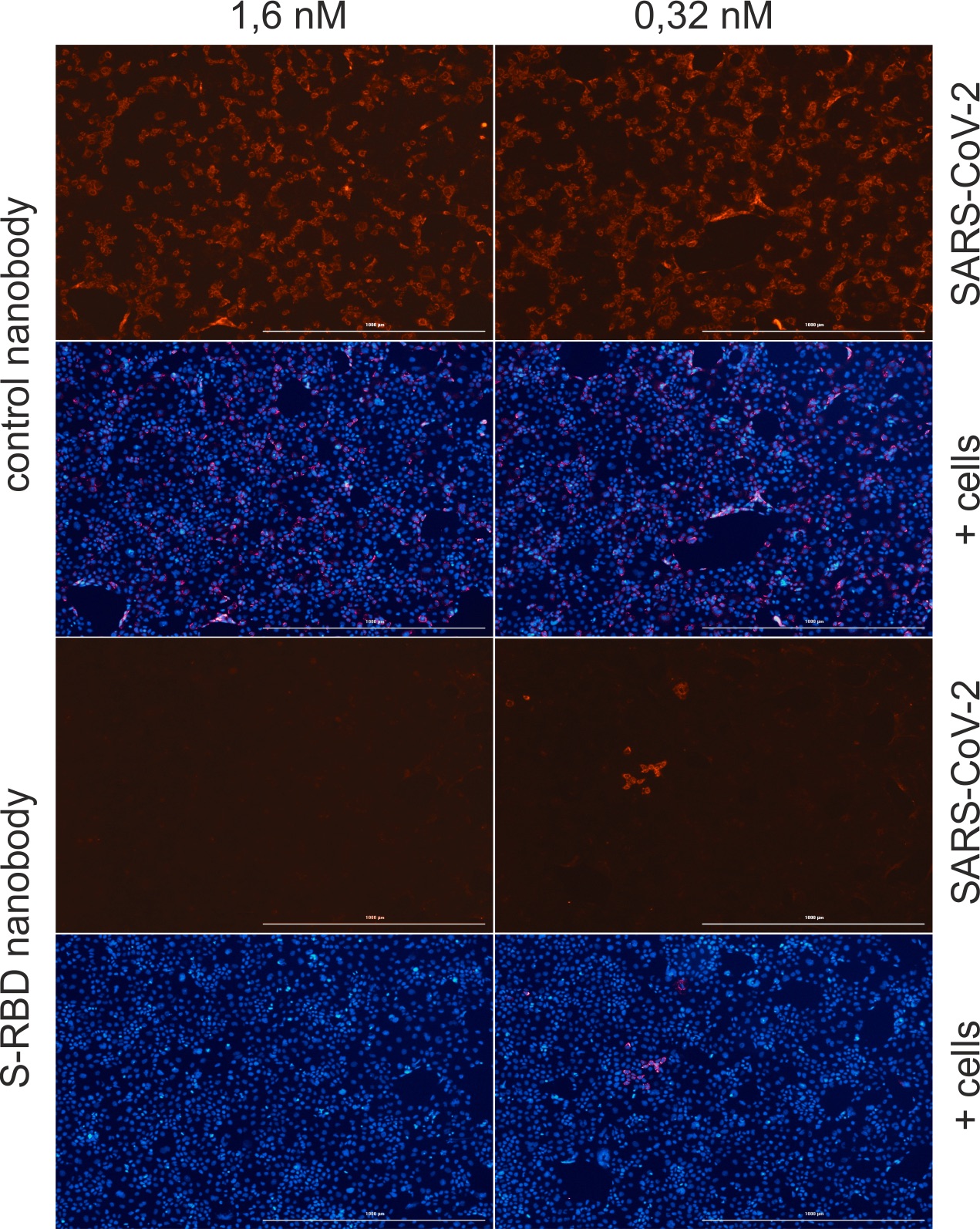 Human epithelial cells were infected with SARS-CoV-2 and simultaneously treated with a control nanobody (Nb) or an Nb against the receptor-binding domain of SARS-CoV-2 (S-RBD). After 40 hours, the nuclei (blue) and the nucleocapsid protein of SARS-CoV-2 (red) in the cells were stained and analysed under the fluorescence microscope. The S-RBD Nb prevents the viruses from entering the cells. © M. Schindler
Human epithelial cells were infected with SARS-CoV-2 and simultaneously treated with a control nanobody (Nb) or an Nb against the receptor-binding domain of SARS-CoV-2 (S-RBD). After 40 hours, the nuclei (blue) and the nucleocapsid protein of SARS-CoV-2 (red) in the cells were stained and analysed under the fluorescence microscope. The S-RBD Nb prevents the viruses from entering the cells. © M. SchindlerIf, for example, the genetic information of a nanobody is introduced into mammalian cells in combination with a fluorescent protein, the host cells form a so-called chromobody that binds specifically to individual structures in living cells and makes them visible. In this way, changes in the cell can be monitored and, for example, the effects of drugs be examined in real time.
Prof. Dr. Ulrich Rothbauer, who has been working in this field since 2003 and has led a research group at the NMI Natural and Medical Sciences Institute in Reutlingen since 2011, is a pioneer in the field of research and application of nanobodies. In summer 2020, his team successfully isolated nanobodies that recognise SARS-CoV-2. They are directed against the receptor-binding domain (RBD) of the spike protein on the surface of the virus, which the virus uses to dock to and gain entry into human host cells. "We came across an unusually large number of nanobody candidates that bind with a high affinity. [...] The RBD is a major target of SARS-CoV-2 antibodies; alpacas, for example, produce a huge number of antibodies after immunisation with this antigen," explains the researcher. Fortunately, this is equally true for humans after vaccination.
Some of the nanobodies found bind directly to the site that the RBD uses to dock to ACE2 (angiotensin-converting enzyme 2), the entry port on human body cells, and can block this interaction. Others recognise an epitope outside this area. Rothbauer's group has now produced a biparatopic nanobody (bipNb) that binds two different epitopes within the RBD and thus has a high neutralising efficacy in vitro.
 Prof. Dr. Ulrich Rothbauer and Prof. Dr. Michael Schindler are cooperating closely to investigate how individual nanobodies function. © U. Rothbauer and M. Schindler.
Prof. Dr. Ulrich Rothbauer and Prof. Dr. Michael Schindler are cooperating closely to investigate how individual nanobodies function. © U. Rothbauer and M. Schindler.Whether or not the bipNb can really prevent infection with the novel coronavirus was investigated with living cells in the laboratory of Prof. Dr. Michael Schindler, head of the Molecular Virology research section of the Institute of Medical Virology and Epidemiology of Viral Diseases at Tübingen University Hospital. Schindler operates one of the few security level 3 biological laboratories in Baden-Württemberg where working with highly infectious pathogens such as SARS-CoV-2, dengue, HIV or hepatitis C is permitted. "We are the contact for industry, every clinic and every diagnostic laboratory within a radius of 100 km. It is unbelievable what we have had to deal with this year," the scientist says of the current situation.
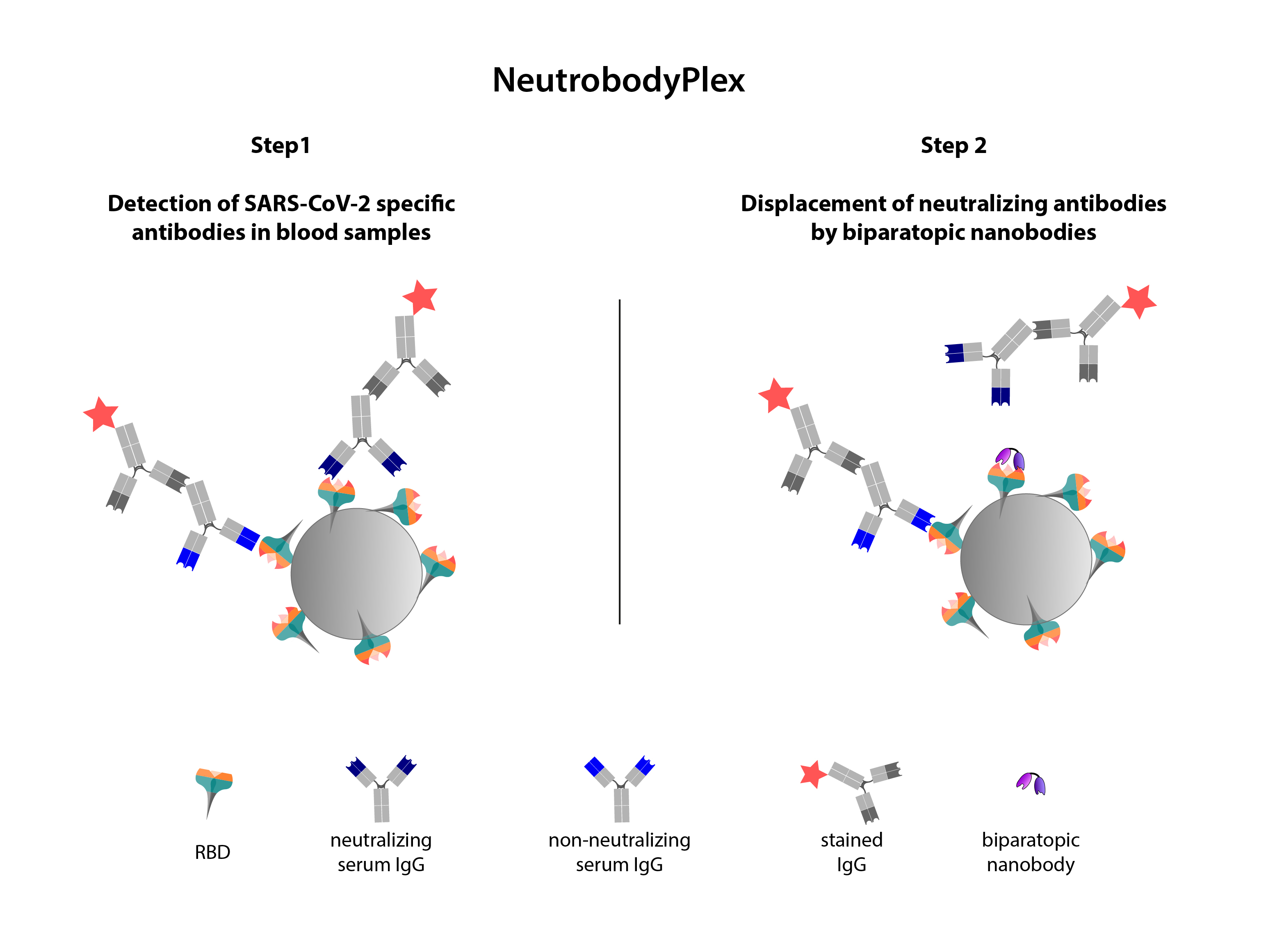 In a first step, the receptor-binding domain (RBD) is coupled to carrier beads. These are then incubated with blood serum so that the antibodies (Ab) present can bind. Their amount is measured using a second, coloured Ab. In a second step, biparatopic nanobodies are added, which specifically displace the neutralising Abs that can prevent the interaction between the RBD and the ACE2 receptor on human cells. The decrease in signal corresponds to the amount of neutralising Abs.
In a first step, the receptor-binding domain (RBD) is coupled to carrier beads. These are then incubated with blood serum so that the antibodies (Ab) present can bind. Their amount is measured using a second, coloured Ab. In a second step, biparatopic nanobodies are added, which specifically displace the neutralising Abs that can prevent the interaction between the RBD and the ACE2 receptor on human cells. The decrease in signal corresponds to the amount of neutralising Abs.
Source: „Schematic illustration of the NeutrobodyPlex”, U. Rothbauer, https://www.embopress.org/doi/full/10.15252/embr.202052325, CC-BY 4.0, edited by R. Menßen-FranzAfter Schindler's group was able to confirm the effectiveness of the bipNbs in the real infection system, Rothbauer's team developed a competitive binding assay, the NeutrobodyPlex. This assay can be used to determine not only the total amount of antibodies against the RBD of the coronavirus in the blood serum of infected or vaccinated persons, but also the amount of neutralising antibodies that prevent an infection. The nanobodies are used to specifically displace only those antibodies that block the interaction between RBD and ACE2. Until now, determining the amount of neutralising antibodies has required an enormous effort and has only been possible in S3 laboratories. The ‘proof of concept’, i.e. the important confirmation that the data obtained in vitro correspond to the results from a living cell system, was provided by Schindler, who is also involved in a large number of similar studies. With NeutrobodyPlex, the quantity and quality of a neutralising antibody response against the RBD can now be easily and reliably monitored. This provides important information for assessing and developing vaccination strategies.
The NMI has already applied for a patent for NeutrobodyPlex, which was largely developed with the financial support of the Baden-Württemberg Ministry of Economics, Labour and Housing. In addition, the assay system has been made available to the Tübingen Centre for Clinical Transfusion Medicine, for example to test sera from staff and patients for the presence of neutralising antibodies. The NeutrobodyPlex binding assay is thus a prime example of application-oriented research.