New study: vaccine therapy for treating patients with chronic leukaemia
Personalised peptide vaccination is expected to improve the treatment of patients with chronic lymphocytic leukaemia. A research team from Tübingen has started a Phase I clinical trial with CLL patients who will undergo ibrutinib treatment. Other leukaemia sufferers as well as cancer patients in general are also expected to benefit in the long term.
Around 30 percent of all leukaemias in Europe are chronic lymphocytic leukaemias, or CLL for short. CLL is a disease of the elderly, with a median age of 72 at diagnosis. In Germany alone, five to six thousand people die of CLL every year. There is still no cure for this type of cancer, but the methods of combating it are now better than ever with new types of drugs such as ibrutinib. However, not all leukaemia cells are eliminated and the cells that are left behind after treatment can reignite the disease process.
 PD Dr Juliane Walz is a specialist in internal medicine and haematology and oncology. She is leading the Phase I clinical trial on personalised peptide vaccination for the treatment of CLL. © University Hospital Tübingen
PD Dr Juliane Walz is a specialist in internal medicine and haematology and oncology. She is leading the Phase I clinical trial on personalised peptide vaccination for the treatment of CLL. © University Hospital TübingenThis is precisely where a novel immunotherapy comes in. It was developed at the University Hospital Tübingen (UKT) in cooperation with the university’s medical faculty. The goal of the therapy is to use a therapeutic peptide vaccine for the targeted elimination of residual cancer cells that are still present after treating CLL with ibrutinib. Development of the vaccine began with the search for tumour-specific surface peptides which only occur on tumour cells and not on healthy cells, due to specific changes in the former. The idea is to synthetically produce these peptides and vaccinate patients with them so that the immune system specifically recognises these structures. The recognition process is expected to set in motion a chain of reactions in which the patient's own immune system cells kill the tumour cells.
What initially sounds quite simple has been a huge challenge for the research team. First, the ‘right’ peptides had to be found. PD Dr Juliane Walz is a senior physician at the Translational Immunology Clinical Cooperation Unit (CCU) at the UKT, which is instrumental in the development of the therapeutic vaccine. Walz is also leading the Phase I clinical trial with the peptide vaccine and reports: "We have been working on isolating and analysing surface peptides from tumour cells for many years. We were eventually able to show for a number of such peptides that they were only found on tumours. This was achieved, among other things, by comparing the peptides with our large database containing information about surface structures on healthy tissues. Further preclinical laboratory tests with donor cells then proved that the peptides are indeed able to activate T cells of the immune system."
World's first personalised vaccine therapy with peptides and adjuvant for the treatment of cancer
However, the researchers from Tübingen were not just seeking to immunise every CLL patient with all the peptides identified. From the word go, the goal was personalised therapy, i.e. using the best peptide mixture for each individual patient because not all CLL patients have the same peptide composition on the tumour cells. However, individualisation also has feasibility limits, as Walz explains: "Theoretically, one could analyse the CLL cells of each individual patient and select his or her exact peptide pattern. However, as things currently stand, this would be time-consuming and costly, rendering such analyses unfeasible. So we have decided to pursue a warehouse principle." This means that the peptides for each individual patient will be selected from a warehouse consisting of peptides that are common in CLL. These are pre-produced in compliance with GCP and GMP guidelines and - based on an individual examination of the leukaemia cells of the respective patient - used in a mixture that best suits the patient’s requirements.
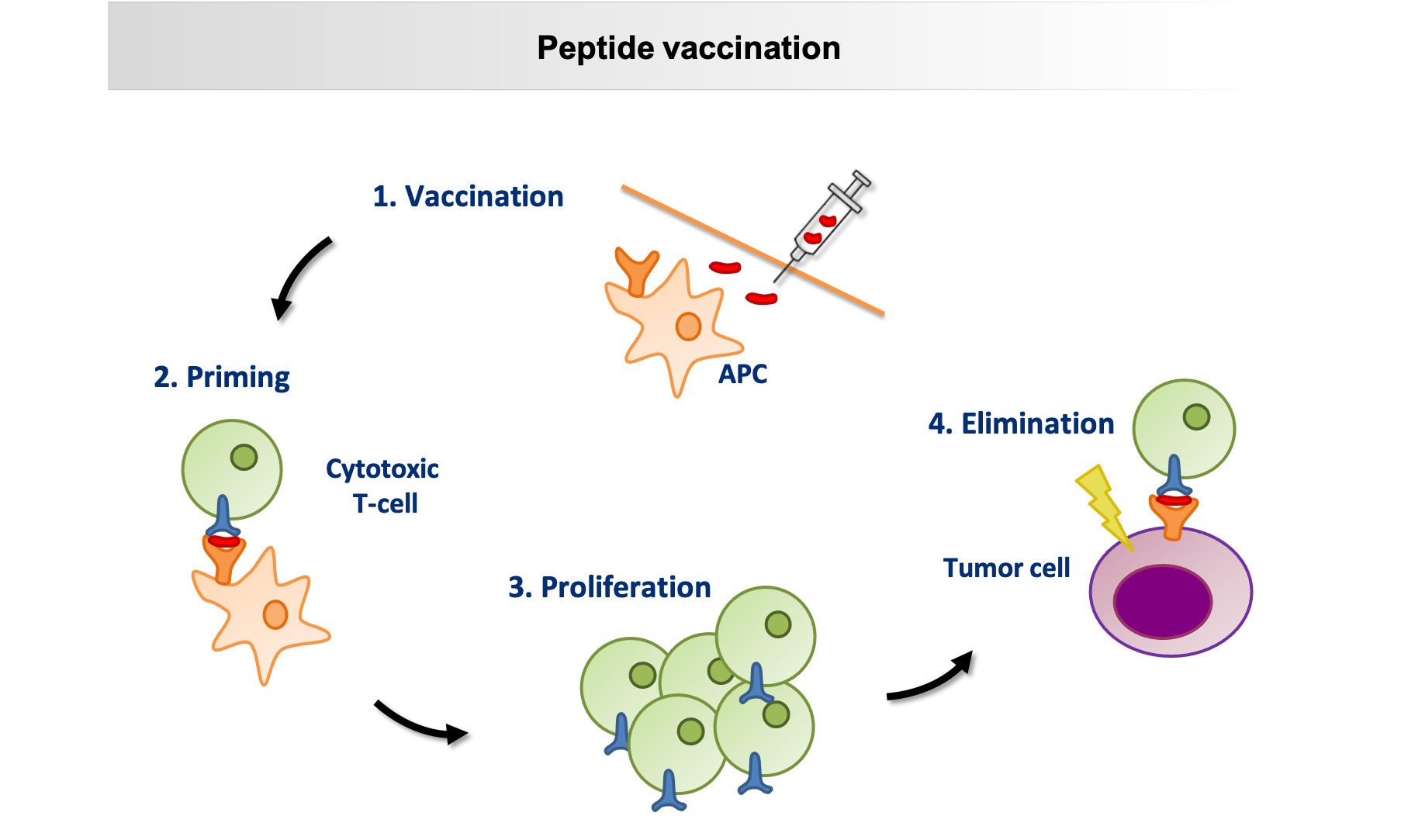 The schematic shows the mode of action of a peptide vaccine: after subcutaneous injection of the synthetic peptides, they are presented to the T cells by so-called antigen-presenting cells (APC), peptide-specific T cells proliferate, recognise the corresponding peptide on the tumour cell and eliminate it. © University Hospital Tübingen
The schematic shows the mode of action of a peptide vaccine: after subcutaneous injection of the synthetic peptides, they are presented to the T cells by so-called antigen-presenting cells (APC), peptide-specific T cells proliferate, recognise the corresponding peptide on the tumour cell and eliminate it. © University Hospital TübingenHowever, the vaccine requires more than that. The concept is that after injection, a depot in the form of a reversible granuloma develops locally at the injection site. From this depot, the immune system is continuously stimulated to discover and destroy isolated CLL cells. This requires an adjuvant, i.e. a vaccine booster, which initially attracts immune cells. The next problem was lurking here, because "good adjuvants for personalised tumour vaccinations were not available until now", as Walz says. The solution came from the laboratory of immunologist Prof. Dr. Hans-Georg Rammensee in Tübingen. In cooperation with Tübingen-based EMC microcollections GmbH, Rammensee’s team developed XS 15, a water-soluble, effective adjuvant that can activate toll-like receptors. These are present in the body as part of the innate immune system. "XS 15 has already been used in individual curative trials with cancer patients and showed a long, strongly sustained T-cell response. Moreover, it is non-toxic and well tolerated," says Walz.
Now that an aqueous solution with vaccine and adjuvant was available, the next challenge was to ensure the depot effect would provide continual stimulation to the immune system. This was achieved with Montanide®, a water-in-oil emulsion, which led to the formation of a granuloma at the injection site. The oils are gradually broken down by the body, causing the granuloma to dissolve.
Peptide vaccination to complement ibrutinib therapy
 The vaccine cocktail is filled into a vaccine vessel. © University Hospital Tübingen
The vaccine cocktail is filled into a vaccine vessel. © University Hospital TübingenThe Phase I clinical trial in humans was commenced after all preclinical investigations had been successful. It is obvious that the vaccine alone will not be able to combat the huge tumour mass of an untreated patient. Therefore, patients in whom the largest part of the tumour burden has already been suppressed with ibrutinib will be recruited to this trial. "Ibrutinib is the ideal drug for us because it targets B-cell lymphocytes and rather than limiting the T-cell response we are targeting, it actually improves it," says Walz. The clinical trial itself is being carried out at the University Hospital in Tübingen and the Robert Bosch Hospital in Stuttgart. Interested participants are still welcome to register for the clinical trial. The recruitment phase will run until mid-2022 at the latest. "The essential prerequisite for CLL patients in this trial is that their treatment plan involves ibrutinib therapy, but has not yet been started. This is because in order to find the optimal peptide mixture, we have to take CLL cells from the patients and analyse them before they receive ibrutinib," says Walz. Cinical trial participants can undergo ibrutinib therapy anywhere, and will only have to go to either one of the study centres in Tübingen or Stuttgart for the planned three vaccinations at four-week intervals.
Any patients interested in participating in the trial can contact the team by email: kketi@med.uni-tuebingen.de.