Inovedis GmbH’s innovative implant
Next-generation minimally invasive shoulder surgery
The treatment of shoulder injuries such as rotator cuff tears is one of the most common orthopaedic interventions. It is therefore quite surprising that the surgical procedure has not changed much for decades - although it is relatively complicated. The Albstadt-based start-up company Inovedis has now developed a completely new surgical technique based on an implant that can be performed simply, safely, quickly and cost-effectively with minimal stress for patients.
Shoulder injuries are common. It is estimated that rotator cuff tears, in particular, affect around 25 percent of fifty-year-olds; it is further estimated that the percentage of patients in their 70s is even higher (50%).1) Most tears occur in the muscles and tendons that stabilise a person’s shoulder, i.e. the rotator cuff. Rotator cuff injuries can be caused by accidents, but in many cases, they are the result of degeneration associated with the ageing processes, i.e. the loss of cartilage substance and the subsequent detachment of the tendon from the bone. Rotator cuff tears often involve the shoulder which tends to be exposed to chronic overuse, and severely restrict the affected person in their everyday life.
 The two founders of the start-up: Lukas Flöß (left) and Dr. Stephan Welte (right) © Inovedis GmbH
The two founders of the start-up: Lukas Flöß (left) and Dr. Stephan Welte (right) © Inovedis GmbHUp until now, rotator cuff therapy has consisted of an operation in which screw-shaped anchors are placed in the bone of the humeral head. These anchors are then used to suture the tendon in place to return it to its original position and allow the area to heal. Although this is done as minimally invasive surgery, the procedure itself is complex and is mainly performed by trained experts using cameras. The suture threads have to be knotted several times in a stable manner, which constricts the tendon, restricts the blood flow and thus makes healing more difficult. This is why re-ruptures (a further complete tear) occur at an alarmingly high rate, affecting up to 70 percent of patients, depending on the breadth of the injury.
In addition to complete rotator cuff tears, i.e. the complete detachment of the tendons from the bone, quite a large number of patients only experience a partial tear (lesion). Up until now, in the case of lesions, what remained of the tendon has needed to be completely separated from the bone so that the partially torn area could be treated using suture anchors.
Start-up from Albstadt develops new technology
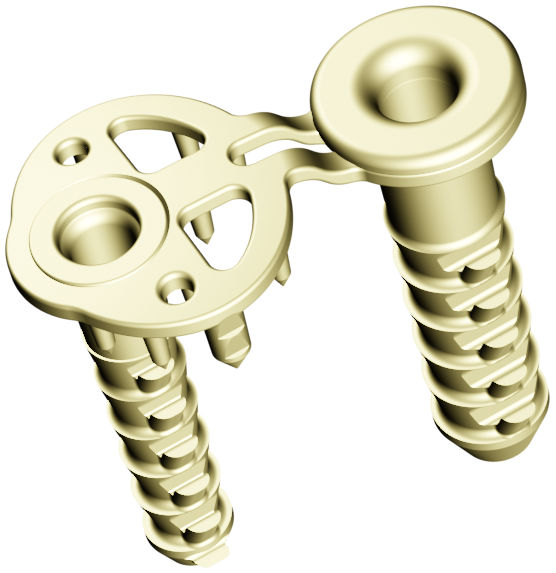 SINEFIX is an implant for the shoulder and consists of two anchors and a base plate with tiny teeth. © Inovedis GmbH
SINEFIX is an implant for the shoulder and consists of two anchors and a base plate with tiny teeth. © Inovedis GmbHThis is far from optimal thought the founders of the Albstadt-based start-up company Inovedis, Lukas Flöß and Dr. Stefan Welte, head orthopaedics physician at the ACURA Clinic in Albstadt, who felt it was high time to come up with something new. "Ideas were not long in coming, and we then turned them into prototypes and tried them out on our own test setups," says Flöß talking about the beginnings of their company. "We were able to apply for patents for the technology back in 2015."
The physicians familiarised themselves with the regulatory framework and started looking for investors. They were successful in their quest. After receiving funds from L-Bank via the BW-PreSeed programme in 2019, they were on their way to setting up their own company. Building on this, the company was able to solidly establish itself in 2021 with seed financing. "First, we were able to convince High-Tech Gründerfonds that our idea was good. Together with MBG Baden-Württemberg and Volksbank Albstadt, High-Tech Gründerfonds then invested 1.6 million euros in our innovation in a first round of financing," says Flöß. "I think that it is a positive sign for our area that part of the financing was granted by regional investors."
Optimal healing thanks to two-dimensional fixation
SINEFIX is the name of the start-up's fingernail-sized innovation and is an implant made of PEEK (polyetheretherketone), a biocompatible plastic. The implant consists of three components: anchors, which are similar to the suture anchors used up to now, and a base plate with tiny teeth that insure the distance to the bone. The implant connects the humerus and tendon firmly to one another over a large area. "This implant does away with sutures and knots by distributing what were previously pressure points caused by the suture thread and the forces on a single surface – the base plate – which provides maximum pull-out force while not crushing the tendon and allowing the blood to flow," explains the Inovedis CEO.
"We have been able to show in studies that both tensile strength and gap formation are much better than those achieved with conventional suture threads. Thus, the tendon adheres stably to the area where it was originally attached to bone. The likelihood of healing is optimal and the mechanical load capacity is significantly better. The implant also provides significant added value in the treatment of patients with rotator cuff lesions, as partially torn tendons no longer need to be completely detached in advance."
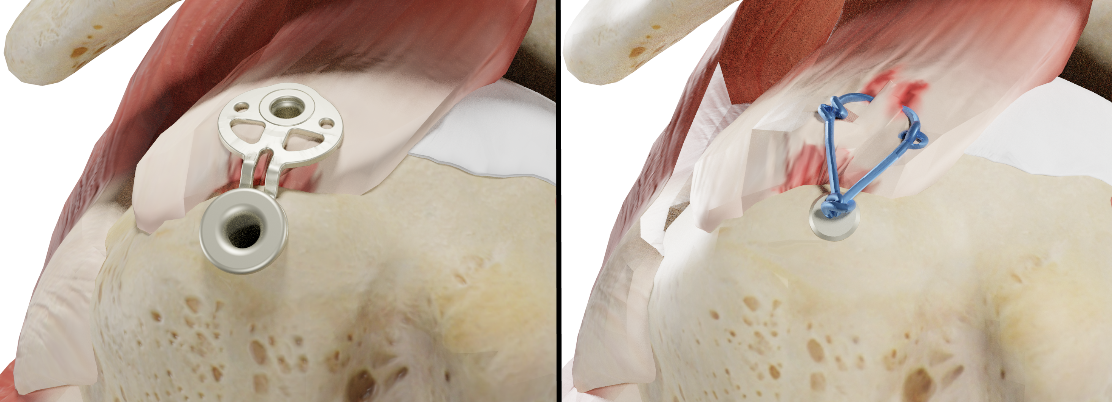 The novel implant (left) replaces previous thread and knot constructions (right) in shoulder surgery and distributes the forces over an area in such a way that blood flow is not impeded, while maximum stability is achieved.
© Inovedis GmbH
The novel implant (left) replaces previous thread and knot constructions (right) in shoulder surgery and distributes the forces over an area in such a way that blood flow is not impeded, while maximum stability is achieved.
© Inovedis GmbH
Easy-to-use and reusable instruments were specially developed to perform the implant procedure. This means that the implant procedure is also safe, simple and can be carried out in the shortest possible time even by doctors who do not regularly perform such shoulder operations. This is good news not only for patients, but also for health insurance companies and clinics, as the expected shorter operation time and better convalescence should make the treatment much more cost-effective.
Approval in the USA, clinical trial in Germany
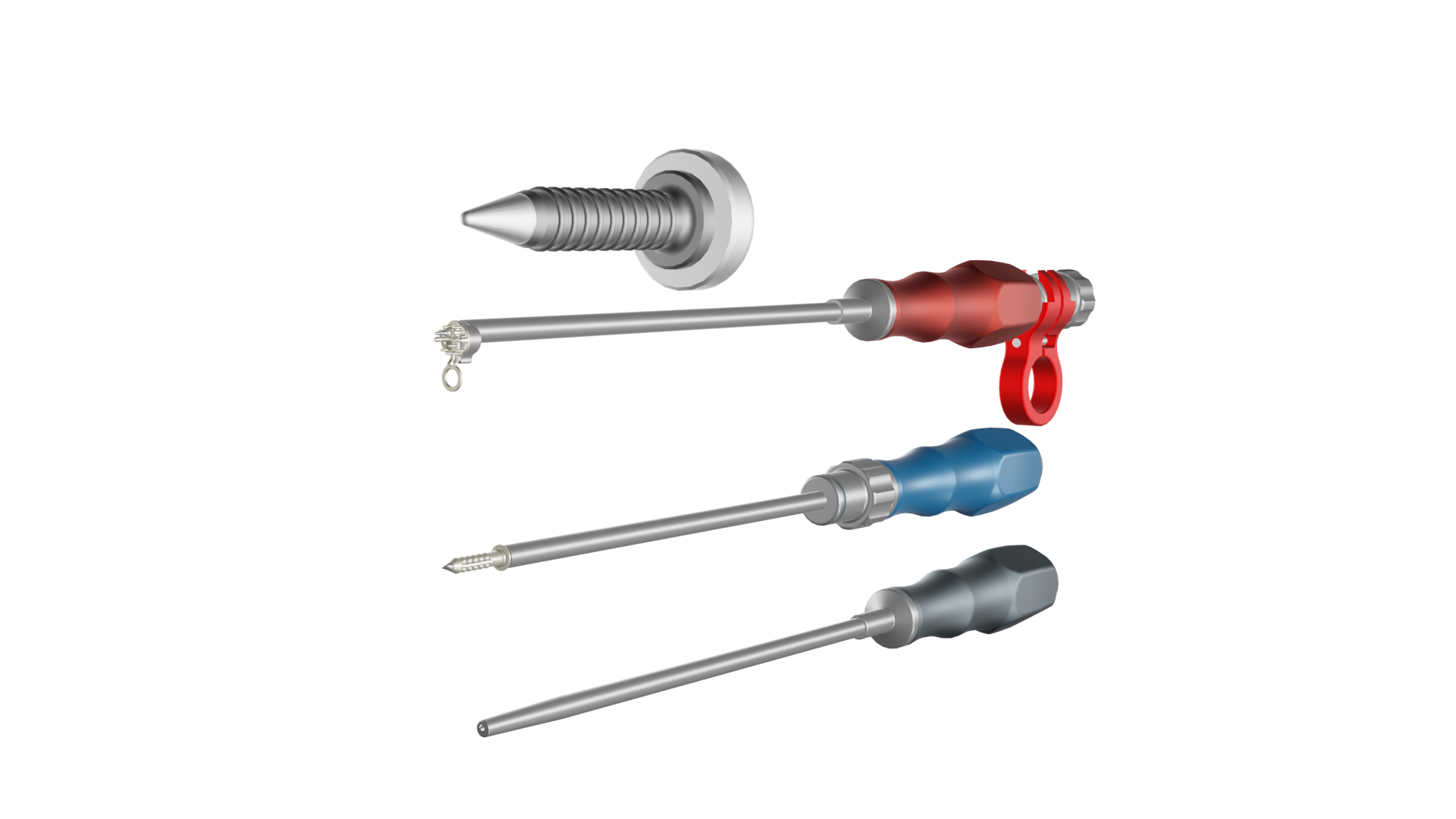 Reusable instruments have also been developed to match the implant, allowing the procedure to be performed safely and quickly © Inovedis GmbH
Reusable instruments have also been developed to match the implant, allowing the procedure to be performed safely and quickly © Inovedis GmbHIn the next few months, the Inovedis team will be seeking approval for their medical product in the USA and starting a clinical trial in Europe. "We were able to apply for a simplified approval procedure [510(k)] with the American FDA back in 2022," says Flöß. "With a 510(k), you don't need to present own clinical data, but you can rely on approved products with the same features, such as the material used, for example." Flöß and his partners are therefore hoping to be given authorisation to sell the implant on the US market soon.
Due to the new European Medical Device Regulation, European authorities require the new product to undergo extensive clinical testing. "That is why we are starting an elaborate multicentre study in the spring to collect clinical data for approval," the medical technology expert reports. "With Prof. Dr. Philip Kasten, who works at the Orthopaedic Surgery Center (OCC) in Tübingen, we were able to attract an internationally recognised specialist to conduct our study."
So it will probably be some time before SINEFIX will be used for treating European patients. "The complicated approval procedure in Europe slows down innovations considerably," says Flöß. "That's why we decided to focus initially on the USA." The implant is produced by a German company, the instruments are made and supplied by a company from Tuttlingen, a city not far from Albstadt.
In addition to the imminent establishment of an American subsidiary, Inovedis is also planning to expand its team over the next few years. Moreover, the Innovedis experts have already set their sights on their next applications.