New method for analysing blood samples
Personalised therapy monitoring for malignant melanomas
Immunotherapy has greatly improved the survival chances of patients with malignant melanoma. A study has now begun at the Department of Dermatology at the University Hospital of Tübingen to develop a way to monitor the course of treatment as effectively as possible. It involves personalised monitoring using liquid biopsies in addition to conventional PET/CT examinations. This analysis procedure of blood samples could enable closer monitoring of patients, leading to the earlier detection of tumour relapse without the need for additional radiological diagnostics.
Malignant melanoma is an extremely malignant tumour of the skin’s pigment cells (melanocytes). As long as the tumour remains in the uppermost layer of the skin, the chances of recovery are very good, as it can be surgically removed. However, if the rapidly growing melanoma breaks through the barrier to the underlying dermis and comes into contact with the blood and lymph vessels, the prognosis deteriorates dramatically. The aggressive cancer cells then migrate through the body and can form metastases in other organs. For a long time, chemotherapy and radiotherapy were the main therapies used at this stage, but they often brought only short-term improvement. However, the possibility of immune checkpoint blockade therapy has existed for nearly ten years now, and has significantly improved the chances of survival. This therapeutic approach aims to activate the body's own immune system to fight the cancer cells.
Checkpoint inhibitors inhibit the brakes of the immune system
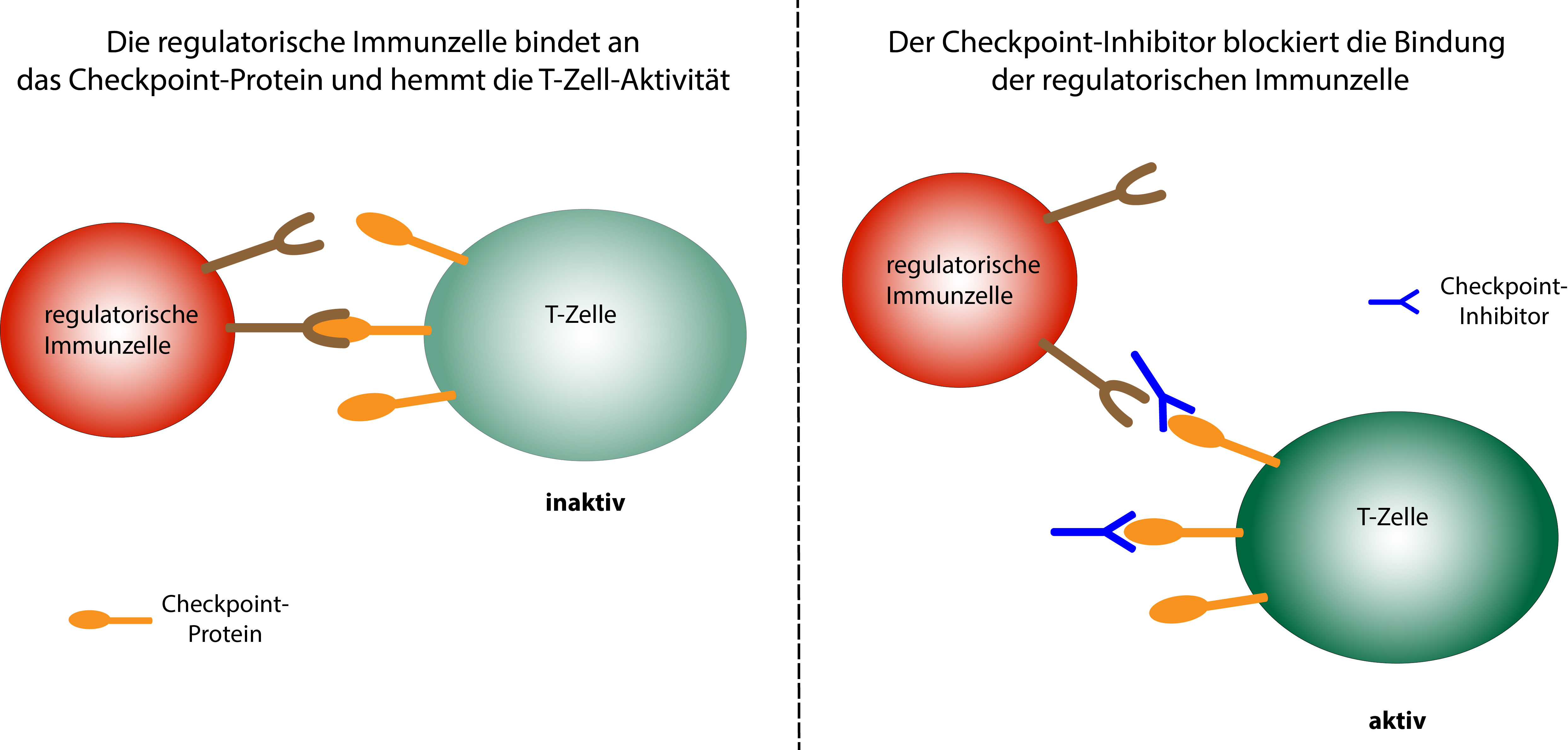 Schematic representation of the mode of action of checkpoint inhibitors © Dr. Ruth Menßen-Franz
Schematic representation of the mode of action of checkpoint inhibitors © Dr. Ruth Menßen-FranzCancer cells have modified protein structures on their surface, which are recognised as foreign by the body's immune cells and trigger an immune response. To prevent the body from reacting excessively with what could be life-threatening consequences, the immune system has a number of inhibitory control points, so-called 'checkpoints', which weaken the immune response with a time delay so that no healthy tissue is destroyed. On the surface of active T-immune cells there are various checkpoint proteins, which, when bound to regulatory immune cells, reduce T-cell activity. Special antibodies also bind to these checkpoint proteins and act as checkpoint inhibitors, blocking recognition by the regulatory immune cells, thus preventing the triggering of inhibitory signals. The immune response therefore lasts longer and cancer cells can be fought more effectively.
 Dr. Andrea Forschner heads the melanoma outpatient clinic at the University Hospital of Tübingen Dermatology Department © Dr. Andrea Forschner
Dr. Andrea Forschner heads the melanoma outpatient clinic at the University Hospital of Tübingen Dermatology Department © Dr. Andrea ForschnerIf different checkpoint inhibitors are administered in combination, as happens when treating metastatic melanoma, the chances of survival increase enormously. More than half of the patients usually respond to the treatment, and in 22 percent of cases, a complete remission with regression of all metastases can be expected. "Immune checkpoint blockade has improved the prognosis tremendously. Five-year survival rates of 60 percent were previously unthinkable," explains Dr. Andrea Forschner, head of the melanoma outpatient clinic at the University Hospital’s Dermatology Department in Tübingen since 2015. "But wherever there is light, there is darkness. With high response rates, you have to keep in mind that more than half of the patients undergoing combination therapy also experience very severe side effects". These primarily include autoimmune inflammation of the bowel with severe diarrhoea, as well as inflammation of the lungs, skin, kidney or meninges. As the fine balance between activation and inhibition of the immune response goes out of control due to checkpoint blockade immune thereapy, the activated immune cells sometimes attack healthy tissue. This can be counteracted with high-dose cortisone, but sometimes the therapy has to be interrupted or even completely halted. Despite the sometimes severe side effects, the long-term chances of success of this treatment approach are enormous.
Liquid biopsy as accompanying detection method
Before starting treatment with immune checkpoint inhibitors and after twelve weeks of therapy, a PET/CT (positron emission tomography combined with computer tomography) examination is performed. This complex radiological procedure displays metastases on the basis of their metabolic activity. At the moment, further PET/CT checks are initially carried out every three months after completion of the therapy, and subsequently every six months. "However, even if patients respond well to therapy, we can see that they [....] are extremely stressed. They don't know whether [the remission] is permanent or whether there will be a relapse. Six months between check-ups is a long time," says Dr. Andrea Forschner, who also trained in psycho-oncology while working in the hospital. For this reason, further procedures for monitoring the success of the therapy would be desirable. A study has therefore begun at the University Hospital of Tübingen which additionally examines the course of therapy using liquid biopsy, a new, highly sensitive method for analysing blood.
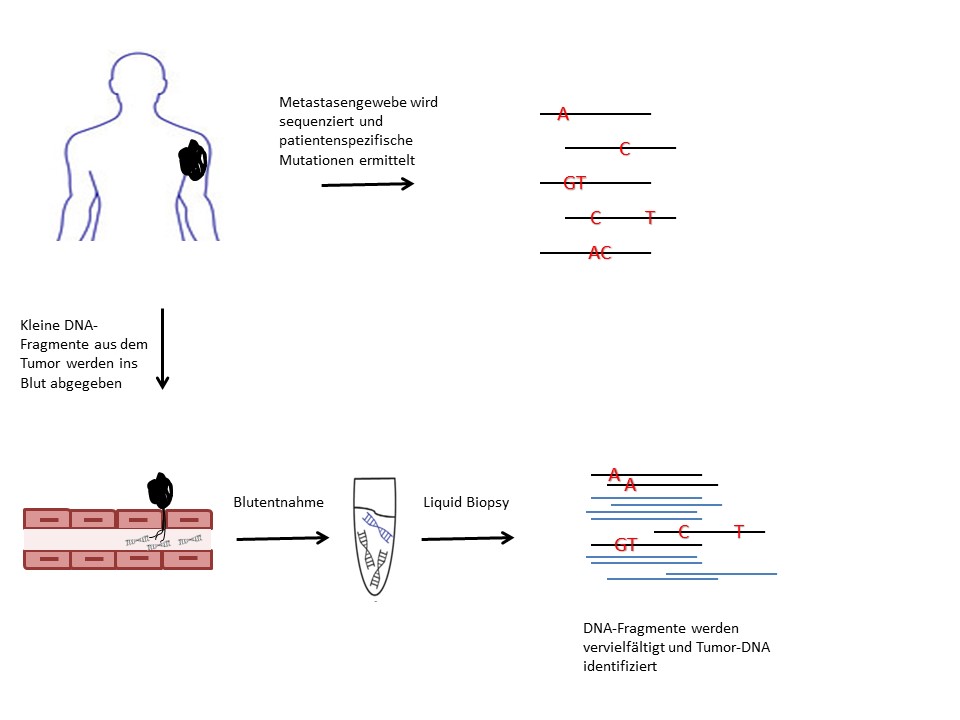 ILiquid biopsies can detect tumour DNA (black) from a blood sample by identifying specific mutations. © Dr. Andrea Forschner
ILiquid biopsies can detect tumour DNA (black) from a blood sample by identifying specific mutations. © Dr. Andrea ForschnerFor the detailed diagnosis of a tumour, a tissue sample (biopsy) is usually taken and examined for tumour-specific characteristics. These primarily include changes in the DNA of the tumour cells, so-called driver mutations, which nowadays are easy to identify using high-throughput sequencing (next generation sequencing, NGS). The tumour releases tiny amounts of its DNA into the blood, which can be detected with liquid biopsies directly through the mutations of the tumour DNA present in a blood sample.
Five to ten individual driver mutations are selected for all patients. A special blood collection tube for the liquid biopsies is filled with blood at each infusion and at each laboratory check during therapy-free intervals, so that the course of therapy can be examined at shorter intervals without placing extra strain on the patient. "We hope that the study will enable us to develop a [monitoring] method that will make patients feel more confident during the therapy-free intervals and enable us to detect recurrences in good time," said Forschner describing the project that she is leading together with Prof. Dr. Christina Pfannenberg from the PET/CT Centre at the University Hospital of Tübingen.
The liquid biopsies are carried out at the Institute for Medical Genetics under the direction of Prof. Dr. Olaf Rieß and Dr. Christopher Schroeder. The procedure is receiving financial support from the Bristol-Myers Squibb Foundation for Immunoncology. If personalised therapy monitoring using liquid biopsies proves successful in treating metastatic melanoma patients, it is hoped that it will become routine clinical practice in the future.