Personalised medicine
Pharmacogenomics enables individualised drug prescription
Every person is unique, and their reaction to medications can be just as individual. For this reason, unexpected side effects occur time and again with common drugs, sometimes with life-threatening consequences. At the Dr. Margarete Fischer-Bosch Institute for Clinical Pharmacology (IKP) in Stuttgart, the influence of hereditary factors on these harmful reactions is being investigated in order to enable individualised therapies.
Adverse drug reactions can occur despite correct dosage and indication; they are a common reason why almost seven percent of patients end up in emergency rooms. The fact that genetic factors are responsible for some of these side effects was already recognised during World War II. It was seen, for example, that haemolytic anaemia occurred more frequently in African-American soldiers who received anti-malarial drugs; this was associated with a deficiency in the enzyme glucose-6-phosphate dehydrogenase (G6PD). This genetic defect is common among people from Africa, the Middle East and South Asia and leads to red blood cells showing increased sensitivity to the drug.
Metabolism of medications via the cytochrome P450 sys-tem
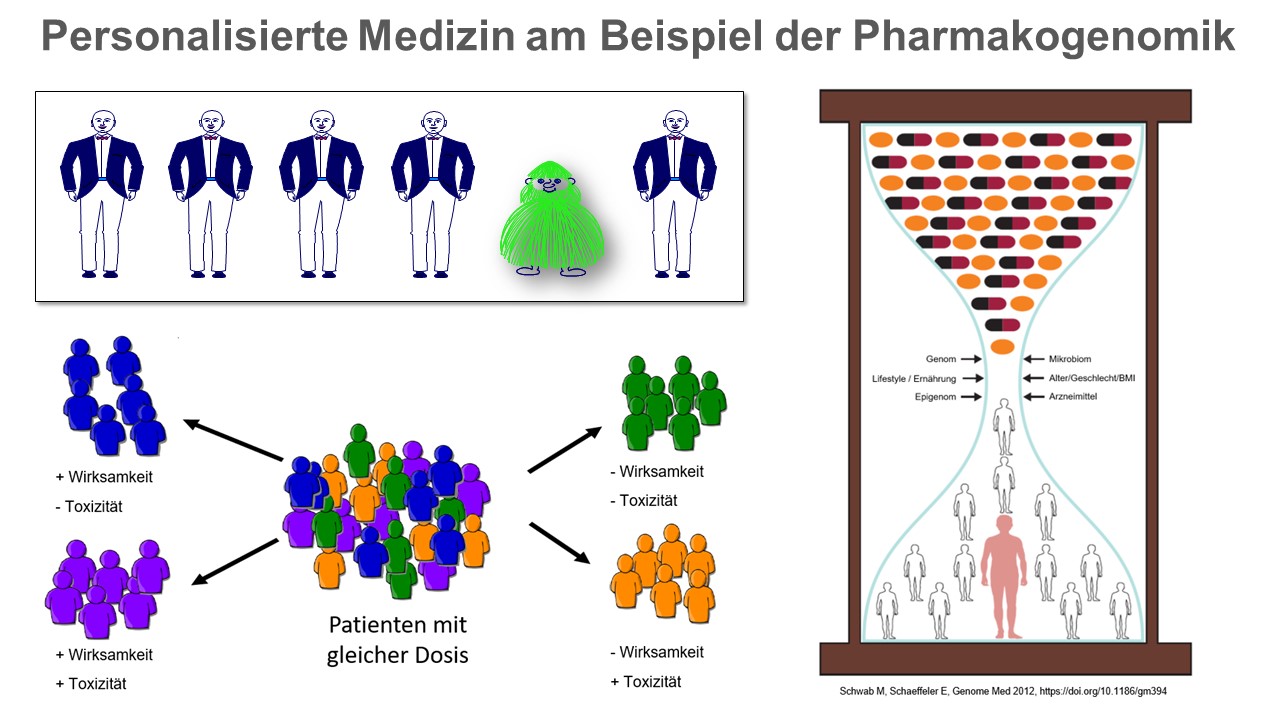 Genetic factors can influence the efficacy and/or toxicity of drugs. Non-genetic factors such as age, gender, life circumstances or comedication also have to be considered. The epigenome (all heritable chemical changes in the DNA and associated proteins) and the microbiome (genome of all microorganisms living in and on an individual) have recently been identified as additional factors that contribute to the interindividual variation in drug response. © IKP Stuttgart
Genetic factors can influence the efficacy and/or toxicity of drugs. Non-genetic factors such as age, gender, life circumstances or comedication also have to be considered. The epigenome (all heritable chemical changes in the DNA and associated proteins) and the microbiome (genome of all microorganisms living in and on an individual) have recently been identified as additional factors that contribute to the interindividual variation in drug response. © IKP StuttgartBased on these findings, the German human geneticist Friedrich Vogel coined the term pharmacogenetics as early as the late 1950s to describe the influence individual gene variants have on the effect of drugs. ADME genes, whose protein products are involved in the absorption, distribution, metabolism and excretion of drugs, play a particularly important role here. They also include the cytochrome P450 system, whose enzymes are responsible for the conversion of up to 80 percent of drugs. Some of these P450 cytochromes have high genetic variability, i.e. different gene variants exist in the population, which result in increased or decreased activity. For affected individuals, this means that they can metabolise some drugs more quickly or more slowly than normal, or possibly not at all.
Some drugs, including many analgesics such as codeine, are converted in the liver from an inactive precursor form (prodrug) to the active form. In the case of a genetically determined protein deficiency or a malfunction of the cytochrome P450 2D6 enzyme, which affects ten percent of the German population, a significantly lower analgesic effect of codeine can be expected, since it cannot be converted into its active metabolite. In such cases, therefore, the focus is not on the side effect of the drug, but on the lack of efficacy, which is also studied by pharmacogenetics.
Gene variants can cause considerable damage
 Prof. Matthias Schwab investigates the influence of genetic factors on drug efficacy. © IKP Stuttgart
Prof. Matthias Schwab investigates the influence of genetic factors on drug efficacy. © IKP StuttgartProf. Dr. Matthias Schwab of the Dr. Margarete Fischer-Bosch Institute for Clinical Pharmacology (IKP) in Stuttgart, who also holds the chair in clinical pharmacology at the University of Tübingen, has been studying the influence of genetic factors on the effect of drugs for many years. "Our scientific investigations are intended to benefit patients and specifically aimed at making drug therapy safer and optimally adapted for each individual," he says, describing his research activities. In the case of medications with a narrow therapeutic spectrum of action in particular, gene variants can cause serious damage. In 2016, he was awarded one of the most prestigious medical prizes in Germany, the Robert Pfleger Research Prize, for his comprehensive work on the effects of an altered TPMT gene (thiopurine S-methyltransferase).
The TPMT gene codes for an enzyme involved in thiopurine metabolism. Thiopurines are used, among other things, as standard medication for treating acute childhood leukaemia, and inflammatory bowel diseases. Due to genetically determined reduced enzyme activity, serious "overdosage" occurs under standard medication in about ten percent of children with leukaemia and consequently leads to bone marrow damage, which in the worst cases can be fatal. TPMT activity is therefore tested in every child with leukaemia in Germany before they begin thiopurine therapy. In addition, Schwab and his team have been able to show that gene variants in another enzyme involved in thiopurine metabolism s, NUDT15 (nudix hydrolase 15), can also lead to bone marrow damage in Europeans receiving thiopurine therapy.
Another focus of the research group is the development of new therapeutic concepts for treating hormone-dependent breast cancer with the drug tamoxifen, whose efficacy depends on the cytochrome P450 2D6 gene variant present.
EU-wide pharmacogenomic study
Hundreds of alterations in various ADME genes are now known to influence the efficacy and/or safety of a drug. The approach taken in pharmacogenomics is broader than in pharmacogenetics and involves the entire genome and the interaction of different genes.
Established in 2017 under the EU's Horizon 2020 research and innovation programme, the Ubiquitous Pharmacogenomics (U-PGx) consortium, of which Schwab is vice-chair, conducted the EU-wide PREPARE (PREemptive Pharmacogenomic Testing for Prevention of Adverse Drug REactions) study, which reviewed the clinical relevance of predictive pharmacogenomic diagnostics in preventing adverse drug reactions. Results of the study are expected in late 2021. Prior to initiating therapy, more than 40 clinically relevant genetic markers that influence the efficacy or occurrence of adverse drug reactions to commonly prescribed drugs were investigated. In half of the patients, depending on the pharmacogenomic result, drug administration was adjusted if necessary, while the control group were given standard therapy. This multicentre study is designed to review the relevance of pharmacogenomic diagnostics in everyday clinical practice with the aim of not only improving therapy, but also demonstrating that the costs of investigations justify their routine use.
Targeted drug development
In some cases, such as cystic fibrosis for example, pharmacogenomics can already be used for targeted therapy. Cystic fibrosis (CF) is one of the most common inherited diseases. Due to a mutation in the CFTR gene (cystic fibrosis transmembrane conductance regulator), non-functional or limited functional chloride channels form in the cell membrane. This leads to the accumulation of viscous mucus in the lungs, but also to changes in other organs. For selected variants of the CFTR gene, targeted drugs that have already been approved are used in the personalised therapy of cystic fibrosis.
However, pharmacogenomic diagnosis still plays a minor role in everyday practice. The PREPARE study mentioned above is intended to provide further evidence supporting everyday clinical practice so that appropriate diagnostics can be implemented across the board.
Rapid development in high-throughput sequencing (next generation sequencing) makes it possible to provide comprehensive, sensitive and specific information about changes in the individual genome within a very short time. In contrast to conventional laboratory tests or genetic analyses for tumour diagnostics, genetic germline variants do not change over the course of a lifetime, so a single pre-emptive diagnostic test is sufficient. In cases where scientific and clinical findings show that changes in ADME genes affect therapy with a particular drug, pharmacogenomics makes it possible to determine the optimal drug dose a patient should be given based on his or her genetic information. This would prevent adverse effects.