Bioinspired technologies
Diagnostics with molecular scissors – is this also possible for on-site COVID-19 tests?
The CRISPR-Cas gene-editing technology is one of the most important developments in molecular biology in recent years. It utilises molecular scissors with which nucleic acids can be cut and edited almost arbitrarily. Researchers in Freiburg, Germany have now successfully used the technology for diagnostic purposes. They are currently working intensively on expanding the system to enable it to detect genome sequences of the novel SARS-CoV-2 virus.
Since 2012, molecular biologists have had access to a molecular tool for changing DNA sequences in a highly specific way. The CRISPR-Cas system, which is based on an enzyme system derived from bacteria, can cut the DNA at a specific place and replace individual nucleotides or entire sequences in the genome, just like an ink eraser. This method is used for research purposes, amongst other things, to specifically switch off genes and thus investigate their function. But CRISPR-Cas also offers great opportunities for patients and consumers, ranging from novel medical therapies to use in plant and animal breeding and pest control.
New task for the molecular scissors
A further option has recently come to light: researchers from Freiburg have developed a method that involves using CRISPR-Cas for detecting short RNA sequences as disease markers, i.e. using the method for diagnostic purposes. “We have been working on a way to examine microRNAs (miRNAs) quickly and easily for several years. We now know that miRNAs are not genetic waste as was originally thought, but instead play an important role in gene regulation and thus in disease processes,” reports Dr. Can Dincer, joint project leader at the Center for Interactive Materials and Bioinspired Technologies (FIT) and at the Department of Microsystems Engineering (IMTEK) alongside Prof. Dr. Wilfried Weber from the University of Freiburg. “We tried a lot of different methods and finally came across the CRISPR-Cas system. The biggest advantage of CRISPR-Cas is that the system can be programmed as required, and you have an entire toolbox to hand for detecting different nucleic acids.”
The requirements for a practical solution for detecting miRNA for clinical purposes were high. A reliable diagnosis using miRNAs can only be established if as many different mRNAs as possible are examined. This is in total contrast to the method used for determining a hormone, which only requires a single value. "In the case of illness, a large number of miRNAs act together, and are either up- or down-regulated, depending on the disease,” explains Dincer. “Our goal was therefore to be able to detect as many miRNAs as quickly and cheaply as possible. We also wanted to implement the method on our existing electrochemical microfluidic system, which is part of a biosensor.”
Reliable detection without any prior nucleic acid amplification
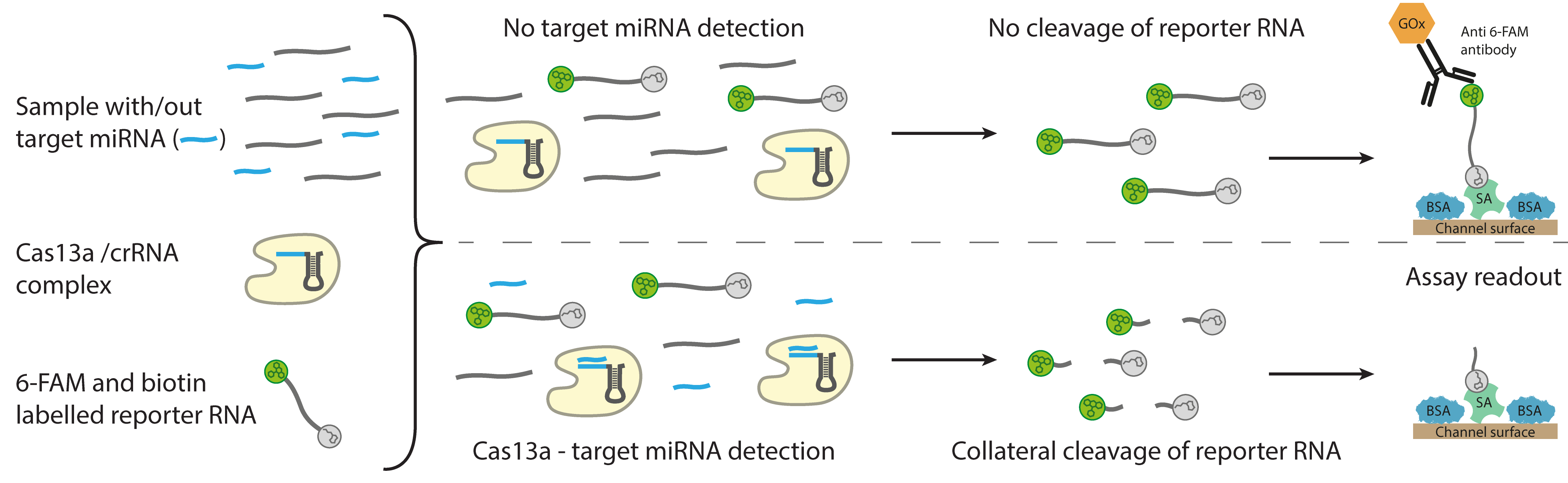 Functional principle of the CRISPR-based assay for the electrochemical detection of microRNA. © Richard Bruch, University of Freiburg
Functional principle of the CRISPR-based assay for the electrochemical detection of microRNA. © Richard Bruch, University of FreiburgqPCR (real-time quantitative PCR) is the current method of choice for detecting miRNA. The researchers also tested this method for use in their biosensor. “However, there is the problem that although qPCR works well with long nucleic acid fragments, it does not work so well and easily with the shorter miRNAs, which are only 18 to 22 base pairs long," says Dincer. “That's why we were looking for alternatives and ended up coming across the CRISPR-Cas13a system. The CRISPR-Cas13a system has many advantages over qPCR: for example, it does not require nucleic acids to be amplified, nor does it require any special equipment or chemicals. This makes the system cheaper and considerably faster than other technologies."
For the diagnostic test, a single drop of serum, which naturally contains many different targets (z.B. miRNAs), is mixed with a reaction solution and droplets are dropped onto the microfluidic chip - the CRISPR biosensor. If a target miRNA is present in the sample being investigated, it will bind to Cas13a in the solution once it recognizes a target-specific, complementary CRISPR RNA. This activates the cleavage ability of the molecular scissors (Cas13a), similar to a key that is turned in a lock to unlock it. The activated Cas13a then cleaves enzyme-labelled reporter RNAs which are also present in the reaction solution. The enzymatic reaction can be measured electrochemically in a glucose-coupled reaction, giving a quantitative readout of the target biomarker – i.e. miRNA – levels in the sample. The strength of the current decreases as the level of the target miRNA increases.
Successfully diagnosing diseases with the CRISPR-Cas13a-powered biosensor
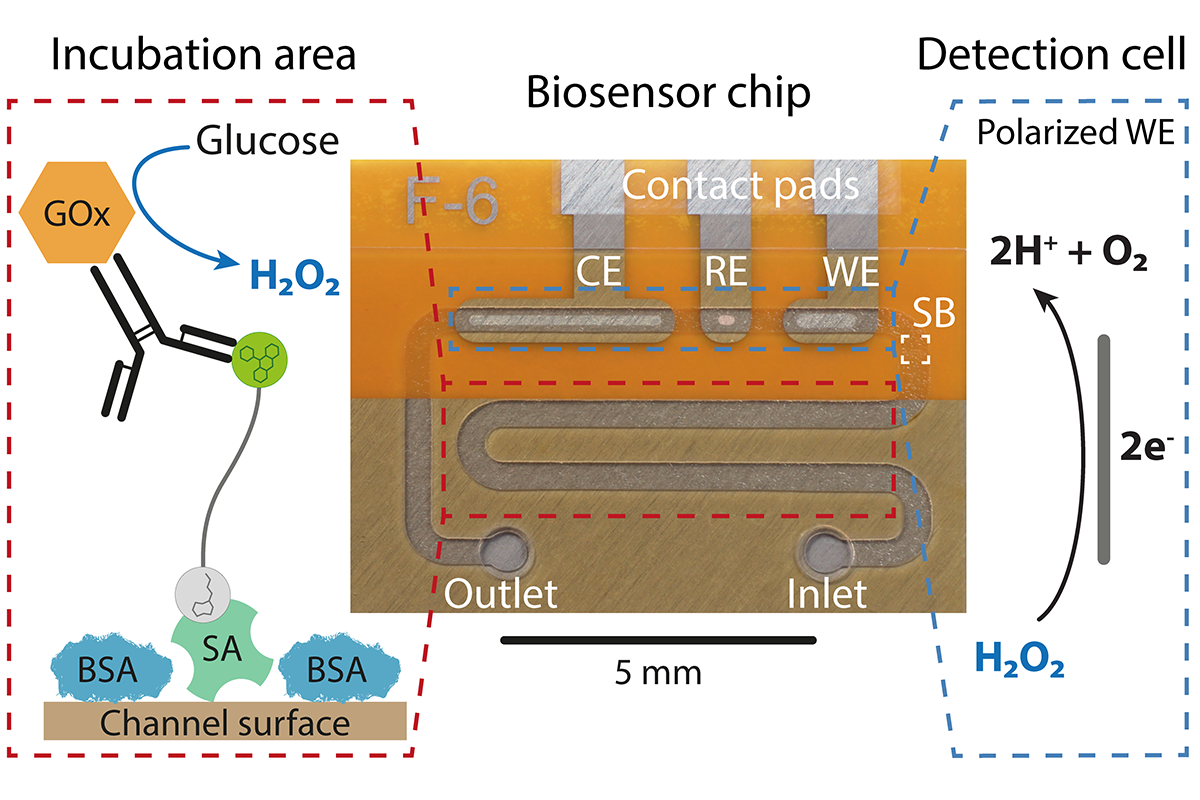 The microfluidic CRISPR biosensor measures a change in current when the sought-after miRNA, and hence biomarker, is detected in the patient sample. © Richard Bruch, University of Freiburg
The microfluidic CRISPR biosensor measures a change in current when the sought-after miRNA, and hence biomarker, is detected in the patient sample. © Richard Bruch, University of FreiburgDincer and his team were among the first to come up with the idea of using the CRISPR-Cas13a system for diagnostic purposes. “When we started our work in 2017, there were maybe only one or two other groups in the world that were working on the same thing,” he says. “It is not that easy to produce the enzyme, you cannot just buy it. This is because there are still a few patent issues that need to be resolved. Incidentally, this is also one of the reasons why the system could not be used for diagnostic purposes before. However, here in Freiburg we are fortunate that Professor Weber and his research group have taken on the biological side of the project and are tailoring Cas13a to our requirements. One of the great things about Freiburg is that there are many opportunities for synergies in this kind of work.”
These synergies have led to a successful outcome. The CRISPR biosensor, which is now ready for application, is the first electrochemical sensor in the world to use the CRISPR-Cas system for diagnostic purposes. "We have already been able to show that this works very well. For example, we were able to successfully detect tumour-specific miRNAs in samples from patients with brain tumours," says Dincer. “The advantage of our system involving CRISPR-Cas is that it is extremely flexible. The chip itself remains the same for all applications. All you have to do is change the molecular scissors’ “lock” to make the chip suitable for a specific application. It is a very attractive and simple solution. We still have to “polish” the “lock” a bit; there is still room for improvement, but in the end the technology will work brilliantly.”
Extension of the system for COVID-19 testing
Dincer emphasises that the practical conditions required for the test are very simple: “You don't need a lot of equipment, because there is no amplification, stirring or shaking involved. All you need is a few hours of incubation time during which the enzyme cuts the gene sequences. And then of course you need the device itself, but it is not very complex and expensive. In principle, we have already done all the development work and are currently building a prototype. We then want to further develop this prototype into a portable desktop device that can be used in doctor’s surgeries for analysing single drops of blood fully automatically.”
However for the time being, these plans have been put on the back burner. The Freiburg researchers are very hopeful that the molecular scissors could be used to carry out some of the enormous number of tests required to identify the novel coronavirus: “We are currently trying to expand our Cas13a-powered measurement system so that it can detect two characteristic SARS-CoV-2 virus genome sequences (COVID-19) simultaneously. The sequences we are working on have been proposed by the Berlin Charité University Hospital for testing purposes,” Dincer reports. “This multiplex approach for analysing a throat swab sample gives us a clear time advantage compared to previous procedures used for this purpose, as, in this case, screening, confirmation and exclusion of a possible SARS-CoV infection take place simultaneously. We are aiming for a total test duration of under 45 minutes. Although similar systems are equally as fast, the advantage of our method is that it works without nucleic acid amplification, thus considerably reducing hands-on time. In future, the test system can also be adapted to all other RNA sequences, making it ideal for keeping pace with potential coronavirus mutations. We will know within a maximum of four weeks whether the system works as we expect it to.
Large-scale Alzheimer’s disease study is being planned
Once development of the novel corona test is completed, the researchers from Freiburg will concentrate on clinical research for the next few years. Amongst other things, they have planned to carry out clinical trials with a large number of patient samples. "The first issue that we have successfully addressed with the biosensor is the diagnosis of certain cancers (*)," says Dincer. "Once the current corona crisis is over, we will start searching for miRNAs that enable Alzheimer's disease to be diagnosed earlier than has been possible with previous disease markers. We will be working with Professor Michael Heneka from the German Center for Neurodegenerative Diseases (DZNE) on this project."
In parallel, the researchers also have plans to place the device on the market within the next few years. They hope to find a large company with the relevant experience, and place the biosensor on the market within the next five years, at the latest. If they cannot find such a company, they are also considering setting up their own start-up company, an option that would take much longer.
Video source: Supplementary video of article "CRISPR/Cas13a-Powered Electrochemical Microfluidic Biosensor for Nucleic Acid Amplification-Free miRNA Diagnostics", published in Advanced Materials (DOI: 10.1002/adma.201905311)
© Ella Maru Studio / University of Freiburg