Novel coronavirus outbreak - a status report on recent developments
Waiting for a SARS-CoV-2 vaccine
Researchers around the world are working frantically on the development of a vaccine against the new coronavirus. However, vaccination is likely to be too late for the first wave of infections that have spread around the world from China since late 2019. A project by the Tübingen-based biotechnology company CureVac, funded by the Coalition for Epidemic Preparedness Innovations (CEPI), uses the company’s technology platform for mRNA-based vaccines to accelerate vaccine development, thus contributing to the future prevention of the disease.
 Coronavirus model © Nilses 2005
Coronavirus model © Nilses 2005Until late February 2020, European countries were hoping that it would still be possible to largely spare them from the new coronavirus epidemic that broke out in Wuhan, a city in central China, in December 2019. The few cases in which SARS-CoV-2 infections had previously been detected in Germany, France and other European countries were isolated, well-defined infection events that could be traced directly to their origin in China, where by February 2020 (according to official data) around 78,000 people had been infected and more than 2,400 people had died from the respiratory disease COVID-19 ("coronavirus disease 2019") caused by SARS-CoV-2. Since February 23rd, when massive outbreaks of the disease were reported in South Korea, Iran and Italy, the dam has burst, and there are few experts who still believe that a global pandemic can be halted. In Germany, too, new SARS-CoV-2 infections have been detected on a daily basis (130 cases by March 2nd 2020). Efforts are being made to quarantine the individuals who have been in contact with infected people and are considered infection carriers. That said, infection chains have proved difficult to trace in the cases reported.
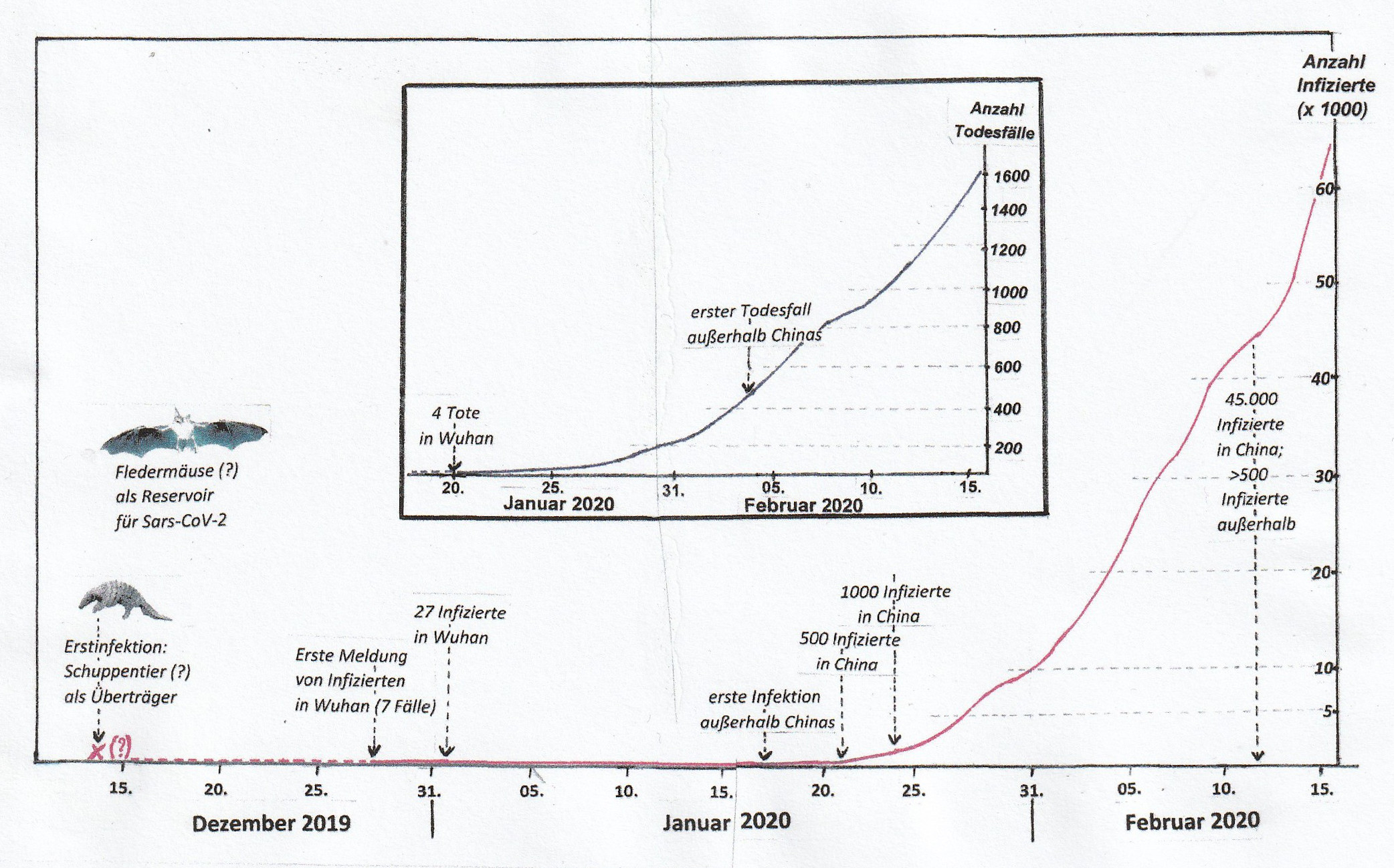 The beginnings of the COVID-19 epidemic. Confirmed cases of infection and deaths from the SARS-CoV-2 virus in mainland China by mid-February 2020. © EJ; figures up until 20th January based on daily press releases, subsequently based on information from Johns Hopkins CSSE, Baltimore, USA.
The beginnings of the COVID-19 epidemic. Confirmed cases of infection and deaths from the SARS-CoV-2 virus in mainland China by mid-February 2020. © EJ; figures up until 20th January based on daily press releases, subsequently based on information from Johns Hopkins CSSE, Baltimore, USA.Efforts to contain the epidemic are being hampered by the fact that some infected people show only weak symptoms of the disease, while others are relatively asymptomatic, but still transmit the viruses. The high infection potential of the novel coronavirus can be explained by the fact that SARS-CoV-2 particles - in contrast to those of its predecessor, the SARS virus from 2002 ("severe acute respiratory syndrome virus") - not only mature and are excreted in the lungs and deep bronchi, but also to a large extent in the throat, so they easily spread through coughing and sneezing. According to Lothar Wieler, President of the Robert Koch Institute (RKI), the highest epidemic authority in Germany, about 80 percent of people affected show only weak symptoms, comparable to the usual annual flu epidemic.
However, 15 percent of those infected become seriously ill, and an average of one to two percent die from the infection, almost always people who are immunocompromised because they are elderly or have an existing illness. So far there have been no deaths from the new epidemic in Germany. To date, COVID-19 has been more aggressive than normal seasonal flu, but the mortality rate is significantly lower than that of the previous coronavirus epidemics, SARS in 2002/03 and MERS (“middle east respiratory syndrome”) in 2012. However, in contrast to COVID-19, these coronavirus epidemics remained largely limited to a specific geographic region and there were much fewer casualties overall.
Infections that cross the species barrier
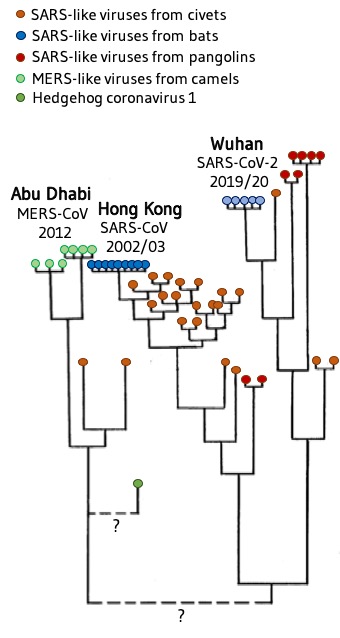 Hypothetical family tree of the beta coronaviruses MERS-CoV, SARS-CoV and SARS-CoV-2 and coronaviruses in other mammals that are believed to be transmit the disease. The length of the vertical lines indicates the approximate genetic distance between them. In particular, the relationship between the MERS virus and the SARS-like virus is unclear. © EJ, modified from https://nextstrain.org/groups/blab/sars-like-cov and https://wwwnc.cdc.gov/eid/article/25/5/18-1534_article
Hypothetical family tree of the beta coronaviruses MERS-CoV, SARS-CoV and SARS-CoV-2 and coronaviruses in other mammals that are believed to be transmit the disease. The length of the vertical lines indicates the approximate genetic distance between them. In particular, the relationship between the MERS virus and the SARS-like virus is unclear. © EJ, modified from https://nextstrain.org/groups/blab/sars-like-cov and https://wwwnc.cdc.gov/eid/article/25/5/18-1534_articleThe virus probably first crossed the species barrier from an animal to a human in early December 2019 on a fish market in Wuhan, where live wild animals are also sold. Phylogenetic tree analyses suggest that a pangolin may have been the carrier. Although these prehistoric mammals are strictly protected, they are frequently traded on the black market in China where they end up in the saucepan or in traditional medicines. In the first SARS epidemic in 2002, civets (cat-like carnivores) were the transmitters of disease, in MERS 2012 it was dromedaries. Bats are considered to be the reservoir from which the animals became infected with coronaviruses.
Neither the number of cases nor the course of the COVID-19 disease cause public panic in themselves. However, health experts and politicians find it difficult to maintain public calm when information about new infections is being reported on a daily basis in the print and broadcast media, when terrifying conspiracy theories are circulating on social networks, and when sinister images of China under siege with its cities in lockdown and epidemic patrols clad in white protective suits are being disseminated - joined now by the cries of alarm from small towns in Lombardy, in Northern Italy. The new coronavirus awakens deep-seated fears, fueled by the memory of devastating epidemics such as the plague, smallpox, cholera and the Spanish flu, which were responsible for humanity's greatest demographic disasters. Added to this is the completely unforeseeable economic cost of the epidemic.
Searching for remedies
So far, no effective drugs are available for the treatment of the lung disease COVID-19. In severe cases, active substances such as interferons, neuraminidase inhibitors and other antivirals, which are also used to combat diseases caused by RNA viruses, are being administered experimentally in severe cases as part of clinical studies. Researchers around the world are frantically looking for new effective therapeutic agents; more than 80 clinical studies were reported in China by mid-February 2020 alone. Not all meet the required scientific standards.
The most important question regarding the prevention of infection with SARS-CoV-2 is: When will an effective vaccine be available? "Unfortunately, that could still take some time," said German Health Minister Jens Spahn after attending a crisis meeting with European colleagues in Rome on February 25, 2020, "even if we do everything we can to accelerate vaccine development." Lothar Wieler says that everything depends on whether we will be able to hold off the worst until a vaccine is available. That is why the authorities are making every effort to contain the chains of infection. "It’s a case of playing for time," said the RKI president. "The more time we have, the more information we can collect about transmission routes and treatment options, and we can prepare ourselves." The objective is to delay the wave of coronavirus infections for as long as possible to stop it coinciding with the flu wave of spring 2020, which would utterly overwhelm health services.
The partnership between CureVac and CEPI
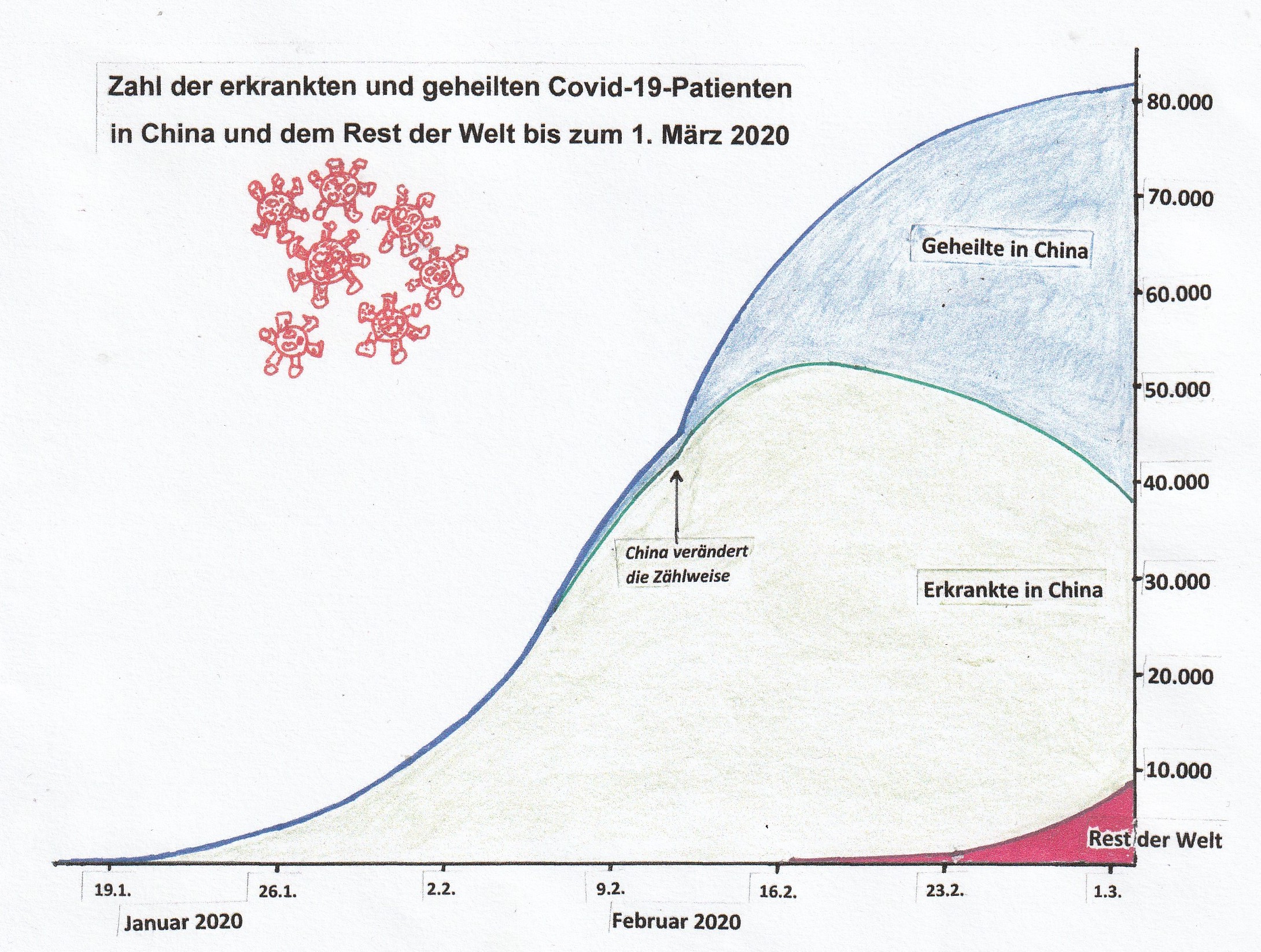 The course of the disease and the number of sick and recovered COVID-19 patients in China and the rest of the world. © EJ, based on information from Johns Hopkins CSSE, updated on 2nd March 2020.
The course of the disease and the number of sick and recovered COVID-19 patients in China and the rest of the world. © EJ, based on information from Johns Hopkins CSSE, updated on 2nd March 2020.In late January, the German government announced that it would provide millions of euros of funding as part of the "Coalition for Epidemic Preparedness Innovations" (CEPI) for developing vaccines against the new coronavirus. CEPI is a public-private partnership of governmental, private, philanthropic and civil society organisations set up in 2017 at the time of the Ebola epidemic. It aims to accelerate the development of vaccines against future epidemics. Early 2019, CEPI entered into a cooperation with the Tübingen-based biopharmaceutical company, CureVac AG, to develop a rapidly deployable vaccine platform based on the company's own messenger RNA (mRNA) technology. CureVac’s platform, which was given 30 million euros of CEPI funding, is a mobile, automated production unit (The RNA PrinterTM) for the rapid supply of mRNA-based active ingredients. A new agreement to develop a vaccine against SARS-CoV-2, signed in January 2020, builds on the existing partnership between CureVac and CEPI. According to Dr. Richard Hatchett, CEO of CEPI, CureVac's mRNA-based vaccine development platform will be used for the new virus type with the aim of “bringing the pathogen’s gene sequence to a vaccine candidate for clinical testing within a few months – which is a significantly shorter time period than appears possible now.” Nobody knows whether this extremely ambitious timeline can be met and whether the partners will succeed; CEPI is therefore also pursuing other SARS-CoV-2 vaccine development projects, including for example, going back to work that began in 2012 and was aimed at fighting the MERS epidemic in 2012, but which was never completed.
“CureVac's technology platform is especially suitable for providing a rapid response to a viral outbreak situation such as the current SARS-CoV-2 epidemic,” said Dr. Mariola Fotin-Mleczek, Chief Technology Officer of the Tübingen-based company. “Thanks to the funding from CEPI, we will be able to develop a vaccine that, once preclinical tests prove successful, could be rapidly tested in humans in a clinical study.” In previous preclinical work, CureVac has already demonstrated that its mRNA vaccines could trigger an immune response against coronaviruses in animals. In addition, new clinical studies with a rabies vaccine show that an immune response can be triggered in humans even with very small amounts of mRNA, as Fotin-Mleczek points out. CureVac's innovative platform, The RNA PrinterTM, also has the potential to ensure the rapid supply of mRNA vaccine candidates against SARS-CoV-2.
A vaccine will probably not become available during the current wave of COVID-19 disease cases, if, as is hoped, it subsides in a few months. Since late February 2020, the number of new infections in the Chinese province of Hubei and the metropolis of Wuhan, which was the first and most affected area, has only been increasing slowly, according to official data. Since the number of successfully treated coronavirus infections that have been cured is simultaneously growing, the current number of affected people is also decreasing. However, no one knows how long the epidemic will remain virulent in this area and how it will develop in other Chinese provinces. The situation in South Korea, Iran and Italy in early March 2020 is similar to that of Hubei a month earlier. It cannot be predicted whether the number of infected people in Germany or France can be better contained. One thing is sure, the vaccine and the experience gained from developing and producing it are urgently needed. It may also be that SARS-CoV-2 has come to stay or - like the influenza virus - to return with the winter season, perhaps in a mutated form, obliging us to react with newly adapted vaccines whenever new flu or SARS-CoV-2 strains emerge.