Biotechnology – driver of innovation in the pharmaceutical industry
The biotech industry is the innovative driver for a pharmaceutical industry that, due to the shift from blockbluster products to personalised medicine, now depends on new concepts. The production of new drugs using genetic engineering relies on knowledge gained from genomics, proteomics and systems biology and creates new treatment strategies that combine therapy and diagnostics (i.e. companion diagnostics) to provide a specific individualised therapy.
The relationship between biotechnology and pharmaceutical companies is a reciprocal one. Around half of the dedicated biotechnology companies in Germany work in the red, in other words, medical biotechnology sector (source: biotechnologie.de). These companies are involved in the development and production of genetically engineered drugs, vaccines and diagnostics. They also provide technology platforms. They are an important driver of innovation and a cooperation partner for the pharmaceutical industry.
According to the biotech report “Medical Biotechnology in Germany in 2013” (Boston Consulting Group and vfa.bio), 385 companies were active in medical biotechnology in Germany in 2012. These companies achieved total revenues of around 7 billion euros. The largest proportion was generated by biotech companies involved in drug development and commercialisation. Far less was generated by biotech companies that provided technology platforms but did not contribute to drug development (580 million euros). The combination of drug development and services (e.g. in the form of analytics) in the field of red biotechnology is a common business model that helps companies cover R&D costs, at least partially, and counteract the difficult financial situation.
Pharmaceutical industry often becomes involved in early drug development phases
Biopharmaceuticals are produced in bioreactors.
© Merck KGaA
The development of new drugs is of major importance for the pharmaceutical industry in particular since the patent protection of many blockbuster drugs is about to expire or has already expired. For this reason, the pharmaceutical industry is increasingly becoming involved in the early stages of development of a new drug by biotech companies. Only recently, CureVac GmbH, a biopharmaceutical company based in Tübingen, signed a cooperation agreement with Sanofi Pasteur S. A., a pharmaceutical company that is specifically focussed on the development of vaccines. The collaboration between the two companies also includes option and license agreements.
On the other hand, the development and especially the approval and market launch of new drugs is a bureaucratically and financially costly process that is difficult for smaller biotechnology companies to carry on their own. They rely on large pharmaceutical companies to which they either license their drug products in late clinical development phases or that acquire the entire company. Examples are Cellzome GmbH and Phenex Pharmaceuticals AG. Cellzome GmbH, a start-up company founded by EMBL in Heidelberg, was acquired in 2012 by the British pharma giant GlaxoSmithKline for a price tag of 76 million euros. Phenex Pharmaceuticals Ag, a dedicated biotech company located in the city of Ludwigshafen, announced in December 2012 that it had entered a cooperation (research cooperation and licensing agreement) with Janssen Biotech, Inc.
Number of genetically engineered drugs is increasing
Approximately five percent of all approved drugs are currently produced using genetic engineering, and this number is increasing. According to the Association of Research-Based Pharmaceutical Companies (vfa), around one third of all drugs in the development pipeline are produced using genetic engineering (source: Forschung für das Leben (Engl.: Research for Life), vfa). In 2012, 93 drugs were in the clinical development pipeline (source: biotechnologie.de).
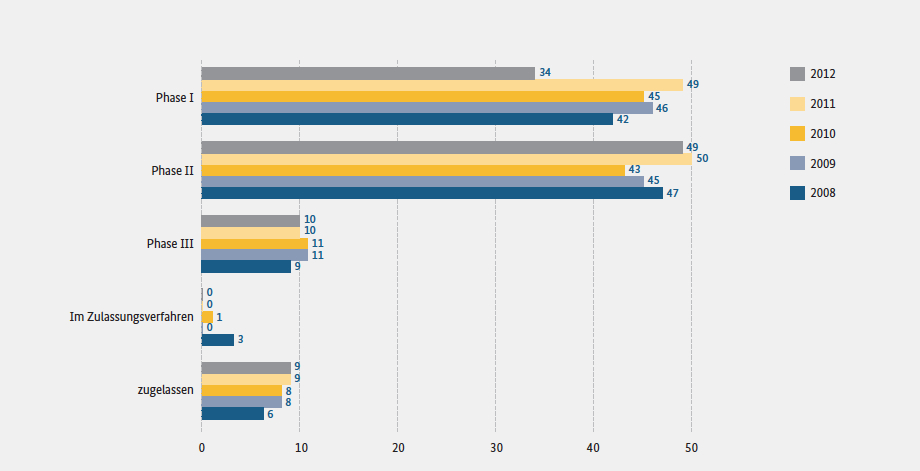 Drug candidates of dedicated biotechnology companies.
© biotechnologie.de
Drug candidates of dedicated biotechnology companies.
© biotechnologie.de
The last drug developed by a German biotech company to receive marketing authorisation was Ameluz®, which was placed on the market by Biofrontera AG in 2011. A total of nine biotech drugs including Ameluz® were placed on the German market until 2012. Equity capital plays a crucial role for biotech drug developers, in particular due to the length of time it takes to develop a drug and due to the relatively high development risk. The financing situation remained difficult in 2012. Although the amount of venture capital increased to 205 million euros in 2012 (source: biotechnologie.de), the money was only invested in a small number of companies. It is therefore crucial for biotech companies to expand their position as drivers of innovation and providers of innovative technology platforms. This means that biotech companies will in future remain to a greater extent than ever key partners to the pharmaceutical industry.
The latest figures for the red biotechnology sector and the pharmaceutical industry in Baden-Württemberg are provided in the industry report entitled “Healthcare Industry 2013 – Data and Facts for Baden-Württemberg”. The new edition of the Biotech Guide provides an overview of the Baden-Württemberg biotech sector, listing 143 concise profiles of biotechnology companies in Baden-Württemberg. Both publications can be downloaded from the respective sections of the BIOPRO portal or through the link on the right-hand side.
CL - 4th November 2013
© BIOPRO Baden-Württemberg