Neurodegenerative diseases
Blood-based biomarkers allow the early prediction of Alzheimer's risks
Most dementia diseases develop insidiously and are only detected at an advanced stage. Researchers at the University of Heidelberg and the German Cancer Research Center (DKFZ) have now identified the glial fibre acidic protein (GFAP) in the blood as a promising biomarker that can be used to determine an increased risk of developing Alzheimer's disease up to 17 years before diagnosis.
The longer we live, the more pathological changes occur in our brain. To a certain extent, restricted functions of the nerve cells and even the death of neurones are normal signs of ageing that cause only mild symptoms. However, if these events occur more frequently, this can result in considerable disturbances of motor functions or serious memory losses.
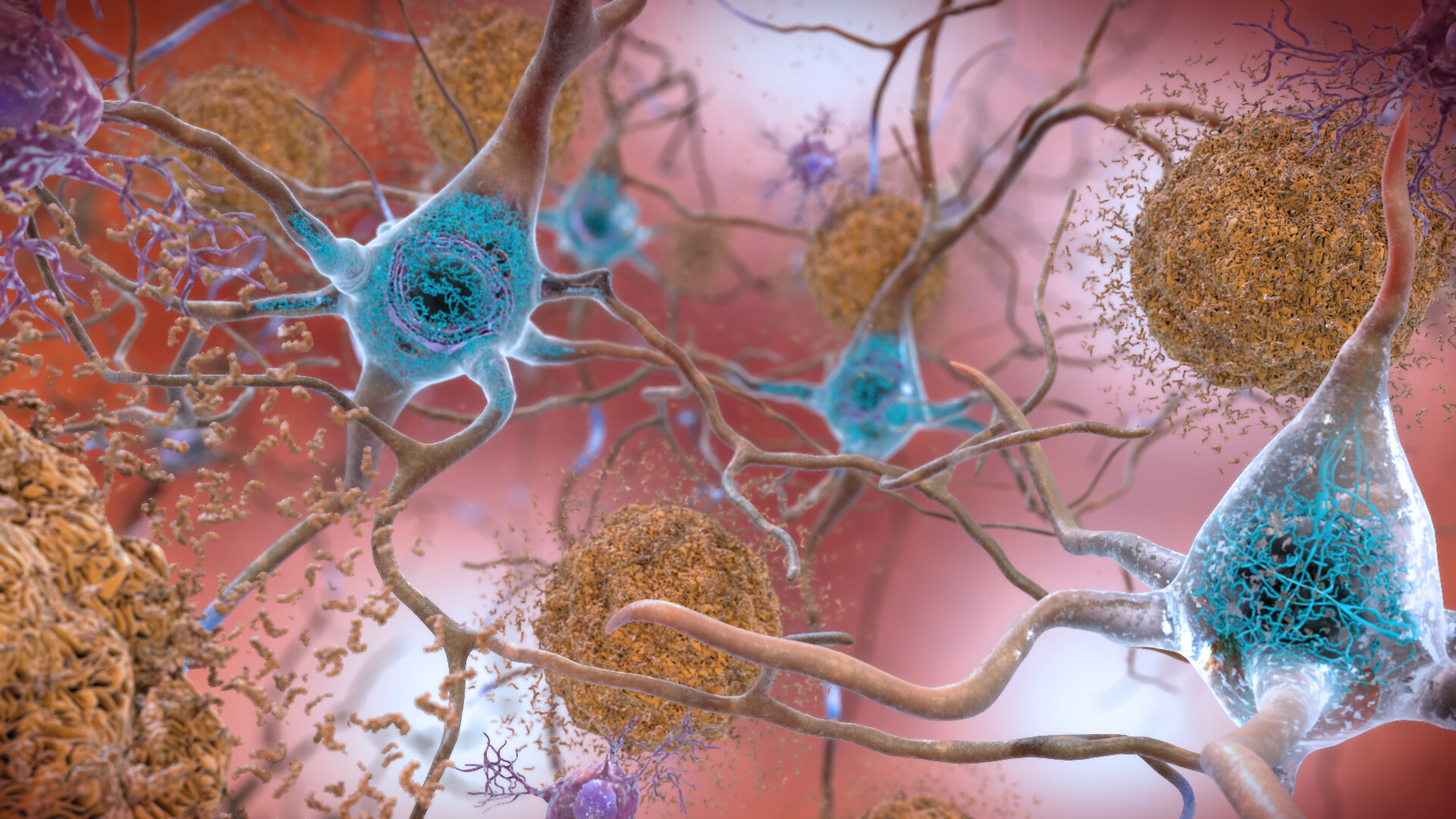 In the brains of people with Alzheimer's disease, beta-amyloid peptides clump together and form senile plaques (brown) in the intercellular spaces. Within the cells, abnormally phosphorylated tau protein aggregates and forms fibrils (blue). Source: flickr.com (2022) I National Institute on Aging, NIH I Public Domain Werk
In the brains of people with Alzheimer's disease, beta-amyloid peptides clump together and form senile plaques (brown) in the intercellular spaces. Within the cells, abnormally phosphorylated tau protein aggregates and forms fibrils (blue). Source: flickr.com (2022) I National Institute on Aging, NIH I Public Domain WerkAlzheimer's disease (AD) is the most common form of dementia, accounting for over 60 percent of all dementias.1) AD is characterised by the deposition of beta-amyloid (Aβ) peptides in the form of so-called senile plaques between the neurones of the brain’s grey matter. These protein fragments are continuously formed during the degradation of the amyloid precursor protein, which is mainly found in the membranes of nerve cells. A change in metabolism with increasing age can lead to Aβ no longer being completely broken down and therefore accumulating. As a result, phosphorylation of the tau protein, which is important for the formation of the cytoskeleton’s supporting microtubules, increases in the nerve cells. In the cytoplasm, this leads to a clumping of the P-tau proteins and the formation of so-called Alzheimer's fibrils. Both protein deposits irreversibly disrupt communication within and between the nerve cells and ultimately lead to the death of neurones (neurodegeneration).
For a long time, pathological changes in the brain could only be detected after the affected person had died, and diagnosis was made solely on the basis of symptoms. Nowadays, it is also possible to determine Aβ and P-tau from the cerebrospinal fluid (CSF) or by visualising the plaques and fibrils using positron emission tomography (PET). However, both methods are complex and expensive and not suitable for widespread application. Moreover, as it is becoming increasingly clear that the pathological processes begin long before the first symptoms appear, a simple, non-invasive method is needed for early AD assessment.
"Our main interest is to find a reliable way to assess the risk of developing Alzheimer's disease that can be easily implemented in clinics," says Dr. Hannah Stocker from the Network Ageing Research (NAR) at Heidelberg University and a scientist in the Department of Clinical Epidemiology and Ageing Research at the German Cancer Research Center (DKFZ) headed up by Prof. Dr Hermann Brenner. For this purpose, she used blood samples from the ESTHER cohort study (Epidemiological Study on the Chances of Cure, Early Detection and Optimised Therapy of Chronic Diseases in the Elderly Population). The ESTHER study has been running since 2000 and is directed by Brenner and the Saarland Cancer Registry.
Blood-based biomarkers for identifying pathological processes at an early stage
The investigations conducted together with the Ruhr University Bochum focused on already established, biologically relevant molecules, so-called biomarkers, which are related to AD. Aβ is not suitable for this purpose because it is difficult to measure and unstable in blood samples. However, information obtained by several independent studies suggests that the version of the tau protein phosphorylated at position 181 (P-tau181) is a promising candidate for early diagnosis of AD and the possibility of distinguishing it from other dementias.2) Since the amount of P-tau181 in the blood correlates with the severity of the disease, and elevated levels of P-tau181 can be detected even before the onset of clinical symptoms, the protein may be suitable for monitoring the progression of the disease or for assessing risk.
NFL (neurofilament light chain) is another marker protein, which so far has mainly been determined in cerebrospinal fluid. NFL is a structural protein found in the nerve cell processes (axons), which is released when cells are inflamed or damaged. However, it is not specific to AD, but indicates all types of neurodegeneration (multiple sclerosis is one example) and is therefore mainly used to classify disease severity. Glial fibrillary acidic protein (GFAP) can also be used as an indicator of dementia-associated changes. It is a component of the cytosolic filaments of glial cells (especially astrocytes), which form the supporting tissue of the nervous system. Elevated GFAP levels in the blood indicate activation of astrocytes and have previously been used as evidence of brain tumours. Recent findings indicate that GFAP levels can also rise due to Aβ deposition.
GFAP is already elevated up to 17 years before diagnosis
 GFAP is already elevated up to 17 years before diagnosis Dr. Hannah Stocker, together with other researchers, identified GFAP as a potential biomarker for Alzheimer's risk prediction. © University of Heidelberg
GFAP is already elevated up to 17 years before diagnosis Dr. Hannah Stocker, together with other researchers, identified GFAP as a potential biomarker for Alzheimer's risk prediction. © University of HeidelbergFor the publication3), which appeared in the journal Alzheimer's & Dementia in March 2022, Stocker and her co-authors determined the amounts of P-tau181, NFL and GFAP in 768 blood samples taken at the beginning of the ESTHER study. They came from women and men who were between 50 and 74 years old at the time, one-third of whom was diagnosed with Alzheimer's during the subsequent 17 years. "We saw that the GFAP levels of those affected were elevated when the first blood samples were taken, even though some were only diagnosed with AD 14 to 17 years later," explains the epidemiologist. "Elevated levels of P-tau181 and NFL, however, only occurred when AD was diagnosed within the first nine years. We did not expect such an early increase and such a clear difference between the markers." The results reveal the biomarker GFAP’s huge potential for risk assessment, which until then had not been so intensively researched.
"However, some questions still need to be answered," explains Stocker, who has been working at the German Centre for Neurodegenerative Diseases (DZNE) in Bonn since October 2022. "So far, it has not been fully clarified whether GFAP is specific for Alzheimer's disease or rather indicates dementia in general." For this reason, in future projects she would like to examine the blood samples of test persons who have developed other dementias. She also plans to analyse samples taken at later dates to learn how biomarker levels change over time. Stocker emphasises: "The ESTHER cohort study is unique, both in terms of its current time frame of 20 years and the number of participants – almost 10,000 subjects at the outset." The long-term goal is to use the biomarkers to identify factors that can influence the time to onset of dementia. This is because some people remain symptom-free for decades despite the protein deposits in the brain.
Even though there are currently no drugs to cure neurodegenerative diseases, early detection of pathological changes offers the opportunity to mitigate symptoms or delay their onset. For the implementation of comprehensive screening procedures, it is important to identify different easily accessible biomarkers so that together they can provide the most accurate information on disease risk. Current data show that GFAP is a promising candidate for this.