CeGat GmbH
Specific coronavirus antibody test for all
Have I perhaps already had COVID-19 in the past? This is a question many of us have been asking. Reliable tests that would provide the answer have simply not been available until now. However, the Tübingen-based biotech company CeGaT is now offering a coronavirus antibody test to anyone who wants one, thus answering this question with the highest probability currently possible. Response to the offer has been huge in the few weeks since the test was launched.
Much about the coronavirus SARS-CoV-2 is new and has turned some of our previous ideas about viral infections upside down. For example, people can be contagious long before they feel ill and many have already had the disease without experiencing any symptoms at all. Knowing the actual number of people infected would be important not only for the individuals concerned, but also from an epidemiological point of view. It is believed that the number of actual infections could be significantly higher than the number of reported infections.
Only an antibody test can clarify whether an individual has already been infected. A blood sample is taken to determine whether antibodies - so-called immunoglobulins (Ig) - have been produced in sufficient quantities against the novel coronavirus. If the test is positive, it can be assumed with a high level of certainty that the individual being tested has already been infected, and is then most likely immune to the pathogen, at least for some time.
However, reliable tests for SARS-CoV-2 antibodies will not be available for a long time. In contrast to the rapid coronavirus tests that work in a similar way to pregnancy tests and have been available for some time, laboratory tests for the novel coronavirus antibodies are much more specific. They have to be in order to rule out positive reactions to other coronaviruses.
Coronavirus antibodies are detected using ELISA
The biotech company CeGaT GmbH in Tübingen, traditionally a provider of genome analyses, has recently joined the ranks of providers of laboratory tests for coronavirus antibodies. "The idea of offering such a test was born out of personal concern", reports Dr. Stefan Griesbach, team leader for sales and marketing. "Our managing directors, Mr. and Mrs. Biskup, tested positive for the virus at a very early stage. Fortunately, they both only suffered mild symptoms; so they set about developing a high-throughput test system that would help to detect past infection."
 Testing can be done directly at the CeGaT premises in Tübingen or at other blood collection centres. However, if the blood sample is taken in centres other than the CeGaT premises, participants need to pre-order a test tube online. © CeGaT GmbH, 2020
Testing can be done directly at the CeGaT premises in Tübingen or at other blood collection centres. However, if the blood sample is taken in centres other than the CeGaT premises, participants need to pre-order a test tube online. © CeGaT GmbH, 2020The actual antibody detection in the test system was developed by EUROIMMUN Medizinische Labordiagnostika AG, a company from Lübeck that specialises in the production of diagnostic tests and which has now also developed an Anti-SARS-CoV-2 ELISA laboratory test. Detection is based on the ELISA method (ELISA = enzyme-linked immunosorbent assay), a long-established procedure in laboratory diagnostics. In the case of the coronavirus antibody test, blood plasma is added to virus-specific particles - the antigens. If antibodies are present in the blood - which is only the case in people who have already had the disease - these so-called IgGs (immunoglobulin G class) are recognised by the virus-specific antigen, bound and cannot be removed by subsequent washing steps. A second antibody directed against human IgGs, coupled with an enzyme, triggers a light signal – a sign that the test is positive.
Specificity is the top priority in this case
"There is nothing new in the test principle itself", explains Griesbach. "The innovation here is the antigen - the SARS-CoV-2-S1 receptor binding domain (RBD). This is a region within the protein in which SARS-CoV-2 differs considerably from other coronaviruses. In addition, our system naturally also includes positive and negative controls. This is because samples occasionally fall between positive and negative. In such cases, however, experience shows that it is best to repeat the test after a few weeks. As far as the coronavirus disease is concerned, antibodies are only detectable two to three weeks after infection, and then the result is usually very clearly positive."
The CeGaT antibody test was primarily designed for specificity and only secondarily for sensitivity. "This is rather unusual for our laboratory", said the biologist. "Normally, our tests are designed to achieve the highest possible sensitivity - in other words, to exclude false negative results. Specificity - in other words, the exclusion of false positive results - is then established manually. But in the case of the coronavirus, specificity comes first, because false positive results can have a fatal effect if the people being tested are lulled into a false sense of security."
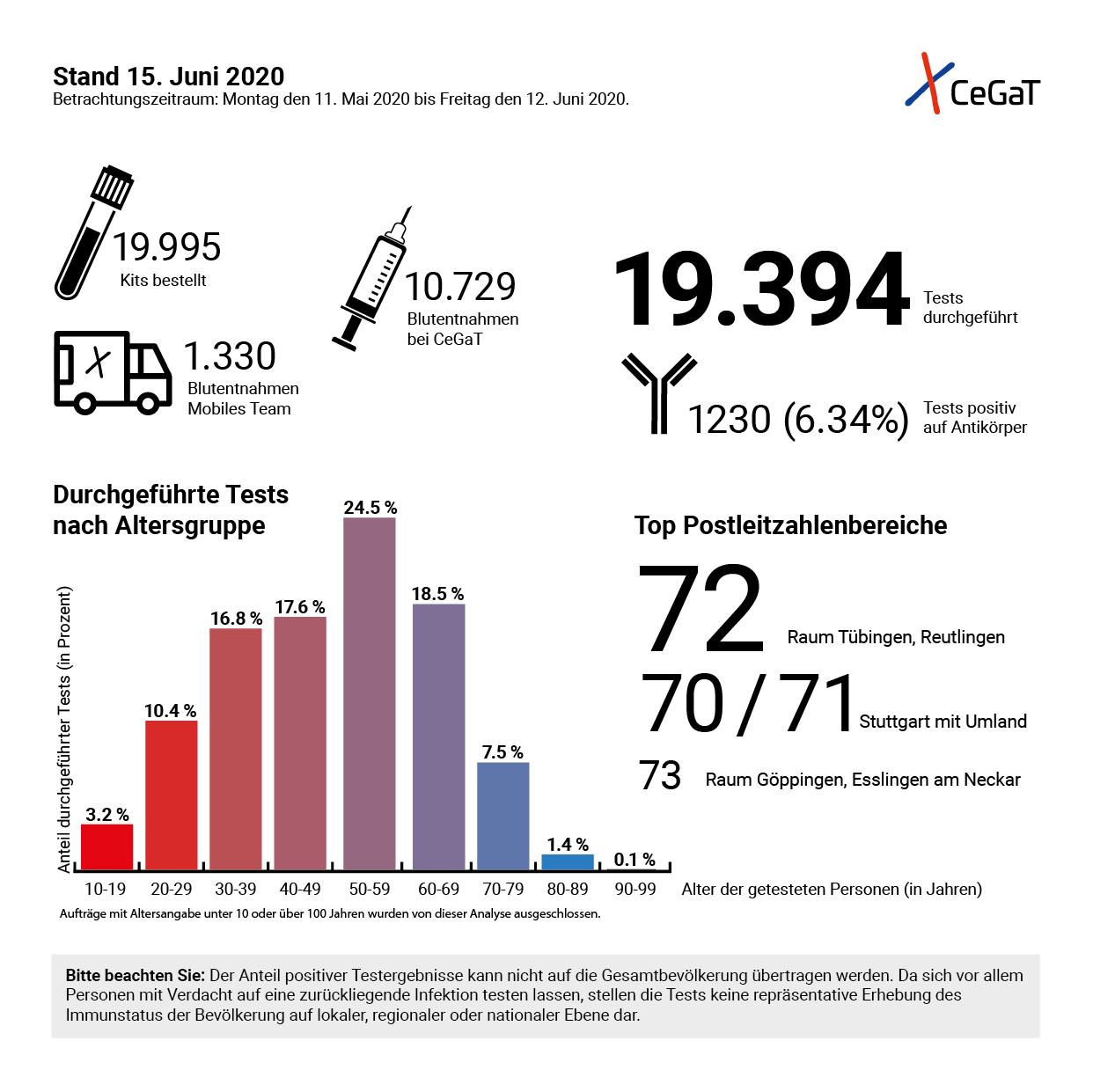 Overview of the coronavirus antibody tests offered by CeGaT © CeGaT GmbH, 2020
Overview of the coronavirus antibody tests offered by CeGaT © CeGaT GmbH, 2020The reason why specificity is so important is that SARS-CoV-2 is not the only coronavirus circulating in Germany. There are many other variants of this pathogen family that are very common and usually only responsible for harmless colds. "In order to differentiate SARS-CoV-2 from the others, our test is directed against a particular structure that most clearly distinguishes the novel coronavirus from the others, the so-called S1 domain of the spike protein on the viral envelope", said the expert. "However, the test - like all other antibody tests currently available - is not accredited as is usually the case with our laboratory, but only has a CE mark and an EUA (Emergency Use Authorisation) from the FDA (Food and Drug Administration). At the moment, this is the only way to do it, but we will carry out interlaboratory surveys as soon as they become possible."
Tests should be widely available and inexpensive
The test from Tübingen was developed and tested with sera from blood donors that were taken and archived long before the first appearance of COVID-19. "This test resulted in a specificity of 99.6 percent, which means that only very few samples tested false positive", said Griesbach. "As we know, the control samples also included samples from patients who had infections caused by other human-pathogenic coronaviruses, and all of these tested negative without exception. However, it is generally the case in medicine that nothing is a hundred percent certain. Anyone who promises something like this cannot be right."
Logistics have been a particularly important issue for the Tübingen-based company as it has been setting up its laboratory for high-throughput antibody testing over the last few weeks. This is because it was essential to offer widely available tests at the lowest possible price - in other words, tests that really are suitable for everybody. "It was important to us to give as many people as possible access to the tests", says Griesbach. "For example, quite a lot of people were rejected for testing in March and April due to lack of testing capacity, even though they were displaying symptoms. In these cases, it is important to know whether they were infected or not."
On-site services to be further expanded
Testing involves having a blood sample taken directly at the CeGaT company premises in Tübingen on weekdays, no appointment is necessary, or ordering a test tube on the biotech company's online system, having a blood sample taken at the doctor's clinic and sending the sample back to the CeGaT laboratory. The results are then returned a few days later by post along with the invoice. In addition, phlebotomy (blood taking) centres have been set up in Stuttgart, Bitterfeld, Dachau and Frankfurt am Main in cooperation with partners.
The CeGaT laboratory is receiving up to several thousand samples a day. "There has been a huge response to our offer", says the team leader. "In the first few days alone, people waited for an hour in the rain to be tested. I think that says it all. By mid-June, we had tested over 23,000 people. We also offer an on-site service for companies with a certain number of employees, which means we come to the company with a mobile team. We want to expand this offer in the near future and are currently in discussion with company doctors and district administrators."