Macrophages interacting with cytomegaloviruses
Cytomegaloviruses subvert macrophage identity
Cytomegaloviruses are basically harmless. However, if they occur along with other pathogens, they can trigger serious diseases. They can manipulate our immune system and encourage normally resident defence cells to migrate. This tends to affect immunocompromised people and babies in the womb. Researchers at the Centre for Chronic Immunodeficiency (CCI) at the Freiburg University Medical Centre have discovered which mechanisms underlie the behavioural changes in macrophages that make it easier for other pathogens to attack.
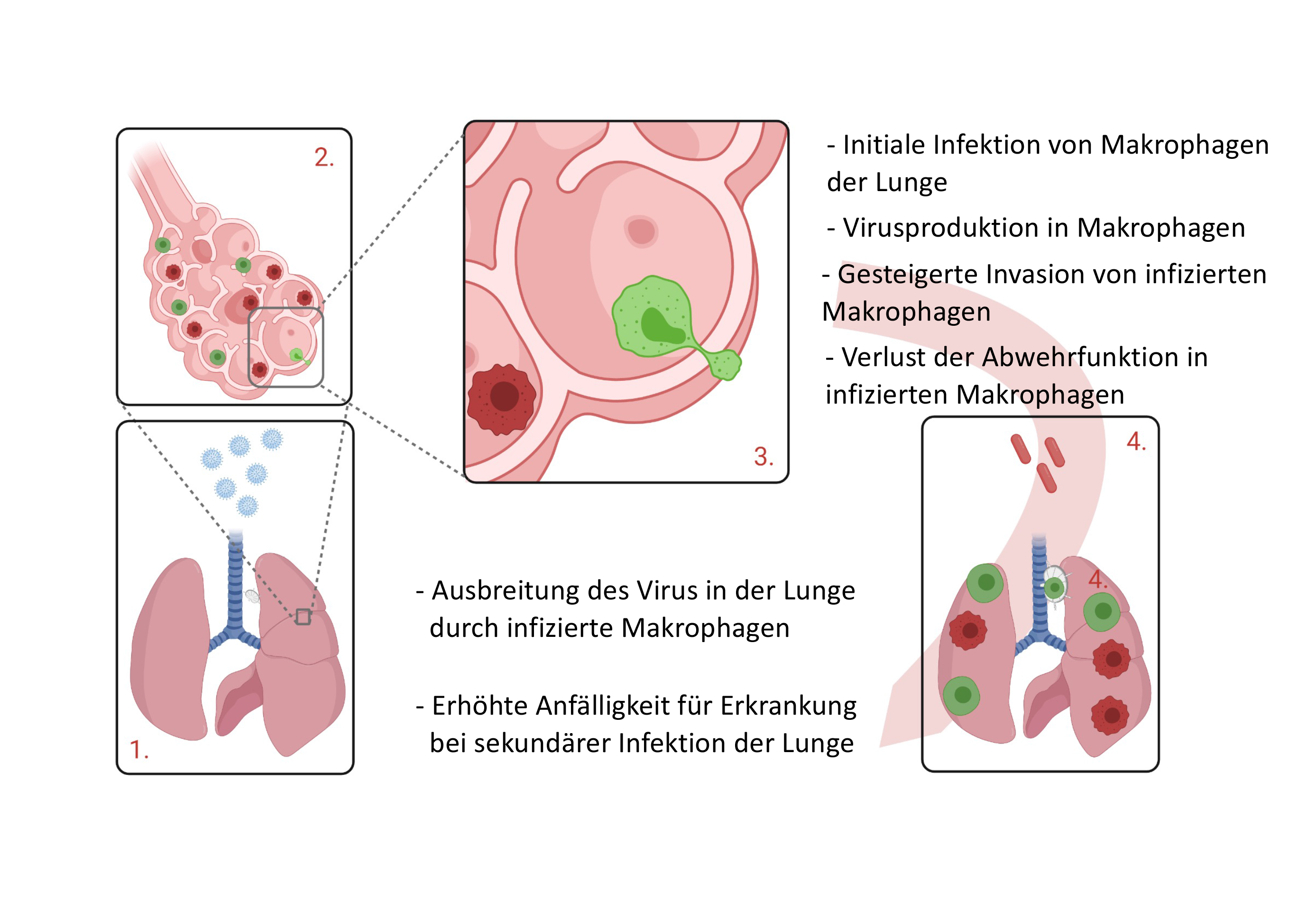
Cytomegalovirus (CMV) is a member of a viral family known as human herpesviruses and is widespread. Cytomegaloviruses have co-evolved alongside humans for millions of years and are virtually part of the human race. It is estimated that around half of the population of Germany carries the virus. Paediatrician and immunologist Prof. Dr. Philipp Henneke has studied the infection mechanisms of cytomegaloviruses and knows that people with healthy immune defences do not become sick due to the virus: "There is extreme adaptation between the virus and humans, evident in the virus’ strong specialisation to humans as hosts," he says. "Human cytomegaloviruses can only infect humans." Infection occurs through close physical contact via body fluids such as saliva, blood and especially breast milk. The virus enters through the intestines and lungs via smear or droplet infection.
Alternating phases of latency and productive infection
 Prof. Dr. Philipp Henneke has discovered how macrophages are manipulated by cytomegaloviruses to ensure they spread. © bz Freiburg
Prof. Dr. Philipp Henneke has discovered how macrophages are manipulated by cytomegaloviruses to ensure they spread. © bz FreiburgInitial CMV infection is usually either asymptomatic or manifests as mild flu-like symptoms such as cough and fever. Nevertheless, it leads to lifelong persistence of the pathogen in the body. "As is typical of all other herpesviruses as well, a so-called latency develops," Henneke explains. "People aren’t aware they have the virus. However, if the immunity situation changes, the virus can start multiplying again."
Normally, a CMV infection clears up on its own and does not require treatment. However, CMV can become active if the immune system is weakened or not yet mature. The risk of foetal damage or miscarriage is particularly high if the mother's first contact with the virus occurs within the first three months of a pregnancy. Newborns who have been infected in the womb can develop growth disorders and neurological damage as well as hearing impairment. CMV is the most common cause of acquired deafness.
However, the baby is not usually at risk if the mother is immune to the virus before pregnancy. Infants and children who are infected with CMV after birth rarely have symptoms. However, in immunocompromised people CMV infection can lead to severe illness. This applies, for example, to patients whose immune system is already compromised by a tumour, an organ transplant or HIV infection. "Cytomegaloviruses play a big role here, and people can become seriously ill," says the immunologist. The virus can infect various tissues such as the liver, intestines, retina and nervous system, but it most commonly affects the lungs. Currently available therapies using antiviral agents can only contain the infection, and are unable to completely destroy the virus.
Change in phenotype and function
 After the cytomegaloviruses enter the lungs, healthy macrophages (dark red) are infected, which then spread further into the lungs and lymph nodes as CMV macrophages (green). © Prof. Dr. Philipp Henneke, Freiburg University Medical Centre
After the cytomegaloviruses enter the lungs, healthy macrophages (dark red) are infected, which then spread further into the lungs and lymph nodes as CMV macrophages (green). © Prof. Dr. Philipp Henneke, Freiburg University Medical CentreAlveolar macrophages in the lungs are the first defence cells to come into contact with CMV during droplet infection. These specialised phagocytes are located in the small alveoli. When allergens or microorganisms enter the lungs, the alveolar macrophages become active, eliminating these tiny foreign bodies by phagocytosis, thus providing the first line of phagocytic defence against microbial invasion in the lungs. If a larger number of microorganisms invades the lungs, alveolar macrophages additionally release messenger substances in the form of cytokines, which attract other immune cells such as neutrophil granulocytes, triggering an inflammatory reaction. "We know that a single alveolar macrophage generally patrols three alveoli," says Henneke. "Alveolar macrophages therefore only reliably move about in their specific territory."
When CMV enters the body via the lungs and infects macrophages, "the macrophages are completely changed by the virus", Henneke says. "Resident tissue macrophages go elsewhere, move much faster and invade other tissues.” As a result, the infected macrophages cause the viruses to spread to distant parts of the lungs and neighbouring lymph nodes, seemingly without any significant response from the body. They lose their ability to phagocytose and produce cytokines and also no longer look like macrophages. The cytomegaloviruses have reprogrammed them completely.
A kind of invisibility cloak for the macrophages
Together with Sebastian Baasch and Dr. Zsolt Ruzsic and their team at the Centre for Chronic Immunodeficiency (CCI) at the Freiburg University Medical Centre, Henneke was able to show that the cytomegaloviruses convert the macrophages into virus factories by undergoing a morphological and metabolic transformation, thus adapting the cellular machinery to the requirements of the viruses.
But how is this possible? Macrophages are a type of immune cell that is normally considered fully differentiated. Their functions therefore no longer change significantly, although they do display some variability or plasticity that allows them to cope with new challenges. However, the newly discovered changes in CMV-infected macrophages far exceeded the known level of plasticity. Previously established boundaries were stretched to such an extent that a new cellular status emerged, according to which CMV is able to interfere with the macrophage’s cellular machinery in such a way that cell differentiation is virtually reverted. The result is a cell that once again has the characteristics of a stem cell and has lost macrophage-specific characteristics. As a result, it can no longer perform its defence function and the way is paved for secondary infections, caused for example by bacteria.
"The cytomegaloviruses alter the immune system for themselves so they can spread effectively, but of course they also alter the way the immune system defends itself against other pathogens if they occur at the same time," Henneke says. He and his team performed kinetic analyses of the macrophages' transcriptome and proteome. "They changed to such an extent that they were unrecognisable as macrophages even when we used molecular methods," he sums up. The gene sets characteristic of alveolar macrophages could no longer be found, and the typical surface markers on the cell were downregulated. Instead, the researchers found upregulated genes that code for the maintenance of stem cell properties. As if the cytomegaloviruses had put a kind of invisibility cloak on the macrophages.
Wnt signalling pathway at the heart of transformation
It appears that CMV intervenes directly in differentiation processes by targeting a specific signalling pathway. The Freiburg researchers identified the transcription factor ZEB1 in the infected cells. ZEB1 induces a signalling pathway that is partly responsible for the transformation of macrophages. This so-called Wnt signalling pathway (Wingless + Int-1) causes increased mobility and invasiveness in the infected macrophages. Wnt is also essential for normal embryogenesis in the womb, a phase of life in which many cells actually still need to be mobile in order to find their defined place. But the signalling pathway has also been found in certain forms of cancer, in which invasiveness can manifest in the form of metastasis. In addition, macrophages reprogrammed by CMV can contribute to the development of breast cancer, since tumour tissue often contains cytomegaloviruses.
Wnt now provides Henneke and his team with a target they can use to develop effective therapies for immunocompromised people, for example after organ transplants. Blocking the transcription factor Zeb1 in vitro can prevent CMV-induced invasiveness of macrophages. Further research will reveal what happens in vivo.