Tumour organoids facilitate drug discovery
Drug screening for children with cancer using patient-specific miniature tumours
Standard drugs often don’t work in children and adolescents with recurrent cancer. Researchers from the Hopp Children's Tumour Centre (KITZ) and the German Cancer Research Center (DKFZ) in Heidelberg have been looking to open up new therapy options for those affected, and have cultivated individual miniature tumours from biopsy samples to test the effectiveness of a variety of drugs within a few weeks.
Every year, over 2,000 children and adolescents are diagnosed with cancer in Germany1). Although the chances of a cure are around 80 percent, malignant tumours are nevertheless the second most common cause of death in this age group after accidents. One third of the young patients have leukaemia (blood cancer), followed by brain tumours (24 percent) and malignant changes of the lymphatic system (lymphomas, 14 percent). Tumours of the soft tissues, the nervous system (neuroblastomas) and the kidneys are also relatively common, each accounting for around 5 percent. Carcinomas, on the other hand, i.e. cancers that originate from the epithelial tissue in the skin and mucous membranes of various organs, are very rare in children, whereas 90 percent of adult cancers result from these carcinomas. Special therapy concepts are therefore required for treating children and adolescents.
INFORM registry for patients with recurrent cancer
 Researchers at the Hopp Children's Tumour Centre in Heidelberg led by Dr. Ina Oehme are testing the effect of various drugs on patient-specific miniature tumours. © Tobias Schwerdt/KiTZ
Researchers at the Hopp Children's Tumour Centre in Heidelberg led by Dr. Ina Oehme are testing the effect of various drugs on patient-specific miniature tumours. © Tobias Schwerdt/KiTZAt the Hopp Children's Tumour Centre (KiTZ) in Heidelberg, a joint facility run by the German Cancer Research Center (DKFZ), Heidelberg University Hospital (UKHD) and Heidelberg University, cancer patients receive comprehensive care and, if necessary, an individual therapy plan. The centre also conducts intensive research into the biology of childhood cancers in order to develop new treatment concepts. One fifth of children affected still die because the tumour cells cannot be completely eliminated using existing therapies. In 2015, the INFORM (INdividualised therapy FOr Relapsed Malignancies in Childhood) registry study was therefore launched. It is a transnational genome sequencing programme for children with cancer that comes under the umbrella of the Society for Paediatric Oncology and Haematology (GPOH) and is coordinated at the KiTZ. "This programme is mainly for children who have had a relapse. The intensive initial treatment often causes molecular changes in the tumour, meaning that standard therapies are no longer effective when the tumour recurs," explains Dr. Ina Oehme, group leader at the KiTZ and deputy head of the Paediatric Oncology clinical cooperation unit. "Genetic analyses can be used to identify molecular targets and develop a treatment tailored to the requirements of individual cancer patients." So far, 2,500 patients from 100 centres in 13 countries have been enrolled in the INFORM registry.
Patient-specific miniature tumours enable drug testing
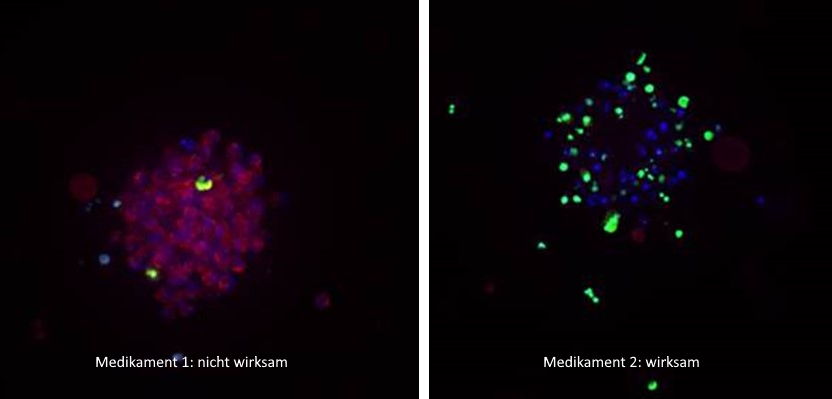 Miniature tumours were treated with different drugs and stained: cell nuclei (in living and dead cells) appear blue; living cells are red, dead cells green. In the image on the left, the drug proved ineffective (red, living cells predominate); the image on the right shows that most tumour cells were killed (strong green; no living red cells). © Heike Peterziel/KiTZ
Miniature tumours were treated with different drugs and stained: cell nuclei (in living and dead cells) appear blue; living cells are red, dead cells green. In the image on the left, the drug proved ineffective (red, living cells predominate); the image on the right shows that most tumour cells were killed (strong green; no living red cells). © Heike Peterziel/KiTZIn addition to carrying out genome analyses, the fresh tissue samples taken in this context were cultivated in the laboratory for a few days and grown into patient-specific miniature tumours. In a study2) published in December 2022 in the renowned journal npj Precision Oncology, Oehme and other researchers from various European hospitals were able to show that these miniature tumours are well suited for testing the effectiveness of many different drugs.
After the biopsy material has been mechanically and enzymatically degraded and red blood cells and free DNA removed, the isolated cells are propagated in serum-free medium for a few days. The latter contains clearly defined factors as also used in tumour stem cell culture, and promotes the formation of three-dimensional cell assemblies, so-called spheroids. Oehme explains: "These miniature tumours reflect the heterogeneity and individuality of the tumour better than conventional two-dimensional cell cultures, which only adhere to the surface of the culture dish. Due to short-term cultivation, the spheroids still contain cells from the original tumour that are enclosed in the tumour structure just as they are in a living organism."
In order to identify suitable drugs, the cells are separated again and a defined number is added to each well of the drug plate, where all active substances are present as duplicates in five different concentrations. They are then cultivated for 72 hours before the metabolic activity is examined to obtain information about the viability of the cells. This is done by determining the concentration of adenosine triphosphate (ATP), which is the source of energy for use at the cellular level. Dead cells are no longer metabolically active and contain no ATP. Cells whose growth is strongly inhibited by cytostatic drugs contain amounts of ATP that are significantly smaller than normal. Comparing the effect of cytostatic drugs with that of control substances provides information on how severely the individual drugs damage the cells. "We are currently establishing an additional microscopic readout in which the spheroid size is measured over time. And we are also developing methods that help us determine the number of killed cells as precisely as possible," reports the cell biologist.
During the two-year pilot phase, which has now come to an end, samples from 65 patients with solid or brain tumours were successfully examined with the help of the miniature tumours. In each case, up to 78 different cancer drugs were tested; both common chemotherapeutic agents with a broad spectrum of effect as well as substances that are specifically directed against certain molecules in the cancer cells. In addition to preparations that have already been approved, others that are currently still undergoing clinical trials were used. At least one effective substance was identified for 47 samples, and in some cases it was possible to identify more than ten such substances.
Individual therapy options
"Molecular biology studies show that the genetic properties of the original tumours are preserved in the miniature tumours," Oehme explains. "Further studies must now be carried out to show how reliably the effect on the miniature tumours mirrors the effect in the diseased persons. This is already under clinical investigation in some cases."
If the results from the culture dish can be reliably transferred to patients, the new procedure could be used to analyse the most diverse types of cancer using small tissue samples to identify individual treatment options within just three weeks. This would open up new chances of recovery for many children and adolescents.