Predicting the success of cancer treatment
Focusing on gut microbiome for CAR T-cell therapy
Cancer immunotherapies use the body's own defences to fight tumour cells. An international consortium of researchers from Germany and the USA led by the DKFZ in Heidelberg has demonstrated that the effectiveness of CAR T-cell therapies greatly depends on the composition of the gut microbiome. The researchers have also developed a model for predicting the long-term response to the treatment.
For quite a while now, immunotherapies have increasingly been used for treating cancer. They are procedures that switch off the tumours’ camouflage mechanisms so that the body's own immune system can recognise and destroy cancer cells.
All nucleated body cells carry HLA (human leukocyte antigen) molecules on their surface, which present peptides from inside the cell. Since every human being has their own individual set of HLA molecules, the immune system is able to distinguish between the body's own cells and foreign ones. If new proteins are produced inside the cell due to an infection or if the body's own proteins change, as is often the case in cancer cells, the peptides presented on the cells’ surface also change. Cytotoxic T lymphocytes normally detect these altered cells with special recognition molecules (receptors) and destroy them. Occasionally, however, individual cancer precursors manage to escape the surveillance system and multiply unnoticed. This can happen when tumour cells no longer present peptides on their surface or when they specifically inhibit the T lymphocytes.
Predictive parameters necessary for complex CAR T-cell therapy
 Led by Prof. Dr. Christoph Stein-Thöringer, researchers from Germany and the USA determine how the gut microbiome influences treatment success of CAR T-cell therapies. © University Hospital Tübingen
Led by Prof. Dr. Christoph Stein-Thöringer, researchers from Germany and the USA determine how the gut microbiome influences treatment success of CAR T-cell therapies. © University Hospital TübingenThe novel immunotherapies address both the ways in which tumour cells avoid being destroyed by the immune system. Checkpoint inhibitors can be used to shield inhibitory tumour cell signals, thus leading to the reactivation of T lymphocytes. CAR T-cell therapy, however, bypasses the HLA-dependent recognition of tumour cells by using genetically modified T lymphocytes from the patient that can recognise tumour-specific molecules (antigens) on the tumour cells. This is achieved by introducing the genetic information of a chimeric antigen receptor (CAR) into the patient’s T cells. CARs consist of an antibody-like recognition domain, the T-cell receptor’s membrane-bound part and a signal-triggering domain in the cell’s cytoplasm. The CAR T cells are then infused into the patient, where they come into contact with and bind to their targeted antigen on a cell’s surface. The CAR T cells are activated, proliferate and become cytotoxic, thus leading to the destruction of tumour cells. Currently, CAR T-cell therapies are used to treat leukaemias and lymphomas caused by degenerated B cells and are directed against the surface marker CD19.
"CAR T-cell therapy is a personalised tumour therapy that is technically very complex and requires skilled staff. This makes the therapy very expensive [ed. note: several hundreds of thousands of euros]," explains Prof. Dr. Christoph Stein-Thöringer, Professor of Translational Microbiome Research at the University Hospital of Tübingen since July 2022. "The extracted T cells are modified in a special laboratory, mostly in the USA, and patients undergo chemotherapy before the modified T cells are infused. Many patients initially respond well to the therapy. However, only about 40 percent benefit from the procedure in the long term. Therefore, methods to find out who the therapy is suitable for and who it is not suitable for are urgently needed. At present, however, no parameters that would provide this information are known. The only information available consists of clinical correlations such as a patient’s age, tumour size or physical constitution."
Gut microbiome influences immunotherapies
Since the CAR T cells must first proliferate and mature in the body, the way they ultimately function heavily depends on the general state of a patient’s immune system. Recent findings show that an effective immune defence is based on an intact gut microbiome, i.e. all microorganisms such as bacteria, viruses and fungi in the intestine. Each person has an individual combination of up to a thousand different microorganisms, which is both genetically determined and dependent on nutritional and environmental factors. Metabolic products produced by bacteria, such as short-chain fatty acids or metabolites of the amino acid tryptophan, not only support the formation of the intestinal barrier but also have a direct influence on T-cell activity. Stein-Thöringer explains: "We already know from clinical studies that patients who have received checkpoint inhibitors or stem cell transplants need to have a healthy gut microbiome in order to be able to respond well to therapy. Checkpoint inhibitors, whose effect depends on functioning T cells will not work so well in patients who have been given antibiotics. Antibiotics create dysbiosis, which is an imbalance in the gut microbial community. We have now investigated the influence of the microbiome on CAR T-cell therapies in more detail."
Led by Stein-Thöringer, who was then a scientist at the German Cancer Research Center (DKFZ) in Heidelberg under Prof. Dr. Eran Elinav in the Department of Microbiome and Cancer, the study involved an international consortium from the University Hospitals of Heidelberg, Munich and Regensburg as well as the MD Anderson Cancer Center in Texas and the Moffitt Cancer Center in Florida. The data1) published in the renowned scientific journal Nature Medicine in March 2023 clearly show that patients who had received a broad-spectrum antibiotic during a period of three weeks prior to the start of CAR T-cell therapy had a significantly lower survival rate. The researchers identified several reasons for this: the fact that these patients had a higher tumour burden and more infections, and as Stein-Thöringer explains, "we also found that antibiotics-induced dysbiosis contributed to these patients dying earlier than those who did not receive antibiotics prior to CAR T-cell therapy."
To better understand the effect of the gut microbiome on treatment outcome, the researchers carried out further analyses on people who had not received antibiotics. This allowed them to identify different species that were either associated with a positive outcome and longer survival times or with a poor outcome. Using machine learning technologies, they then developed a predictive model for long-term response to CAR T-cell therapy based on the gut microbiome. Stein-Thöringer reports: "The process was rather complicated and initial models involving all study participants failed because the gut microbiome of patients treated with antibiotics is too dysbiotic. A new approach was taken that excluded these patients, and this proved crucial. It allowed us to identify a reliable microbiome signature; the predictive model then worked in both the German and American patient cohorts."
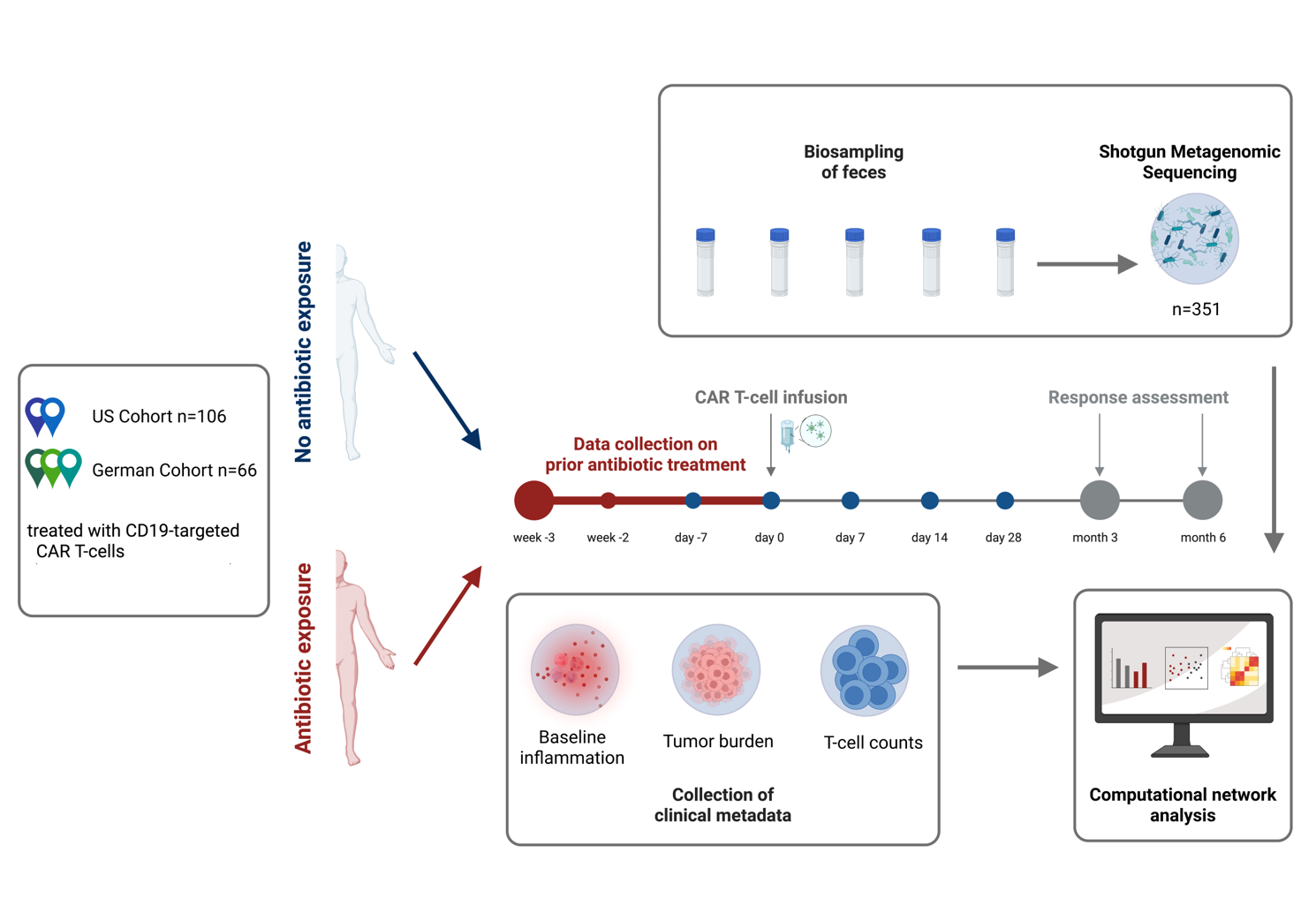 In three German and two American centres, 66 and 106 subjects, respectively, received CAR T cells directed against CD19. For the evaluation, the clinical data were recorded and stool samples were taken at regular intervals before, during and after the CAR T-cell infusion and the intestinal microbiome was analysed using shotgun metagenomic sequencing. A prediction model was created based on the German subjects who were not treated with antibiotics and this was validated in the US cohort. © C. Stein-Thöringer, UKT and V. Blumenberg, LMU Munich
In three German and two American centres, 66 and 106 subjects, respectively, received CAR T cells directed against CD19. For the evaluation, the clinical data were recorded and stool samples were taken at regular intervals before, during and after the CAR T-cell infusion and the intestinal microbiome was analysed using shotgun metagenomic sequencing. A prediction model was created based on the German subjects who were not treated with antibiotics and this was validated in the US cohort. © C. Stein-Thöringer, UKT and V. Blumenberg, LMU Munich
New treatment concepts conceivable
The research, funded by the Mark Foundation for Cancer Research and the BW Stiftung foundation, further shows that certain gut bacteria correlate with a higher number of T lymphocytes in the blood even before therapy begins. In these cases, it is possible to harvest more cells for CAR T-cell therapy. Furthermore, the CAR T-cell product is also better than in those who suffer from dysbiosis. "Our data clearly demonstrate the influence of the gut microbiome on the cytotoxic immune response," Stein-Thöringer says. "That is why we are already working on identifying the functional interactions between individual bacteria species and the CAR T cells. We hope that in the future it will be possible to create the most favourable conditions using immunomodulatory metabolites or special probiotics prior to CAR T-cell therapy. Antibiotics should in any case only be given sparingly and selectively. That said, tumour patients often suffer infections and require antibiotics treatment. My research group is therefore also focusing on finding ways and means to mitigate the harmful effects antibiotics have on the gut microbiome.”
If Stein-Thöringer and his colleagues are able to substantiate their findings in further studies, concrete measurement parameters will be available for predicting the effectiveness of CAR T-cell therapy in individual patients. Moreover, promoting the growth of certain microorganisms in the gut will contribute to significantly increasing the chances of successful treatment.