Affimed GmbH
"Innate cell engagers" to fight cancer
In the fight against tumour cells, a company called Affimed GmbH from Heidelberg, Germany relies entirely on the weapons of the innate immune system. Affimed has developed special innate cell engagers, which are bispecific antibodies that recognise tumour cells and bring them together with natural killer cells and macrophages of the innate immune system, which then kill the tumour cells. Clinical trials using the AFM13 molecule have shown this to be a very promising approach with a favourable safety profile thus far.
Current cancer immunotherapies focus on activating the adaptive immune system’s cytotoxic T cells, which recognise specific structures (antigens) on the surface of tumour cells and then destroy them. In the past few years, this strategy has led to considerable success. However, excessive immune reactions have also caused serious side effects.
Cells of the innate immune system, for example natural killer cells (NK cells) and macrophages, use a different principle to destroy intruders. These immune cells are constantly active, monitoring the body's own cells in search of general cellular changes. The surface structure of healthy cells sends inhibitory signals to the NK cells, thus preventing the healthy cells from being attacked. The surface profile of tumour cells, however, is greatly altered; tumour cells can therefore be recognised and destroyed. Nevertheless, in some cases tumour cells manage to hide from the immune cells (immune evasion). Initial studies have now shown that the targeted connection of tumour cells with cells of the innate immune system can effectively eliminate these "invisible" cancer cells.
AFM13 recruits cells of the innate immune system
 Dr. Adi Hoess has been the CEO of Affimed GmbH since 2011. © Affimed GmbH
Dr. Adi Hoess has been the CEO of Affimed GmbH since 2011. © Affimed GmbHAffimed, a company founded by Prof. Dr. Melvyn Little in 2000, originally focused on the production of traditional recombinant antibodies. Over the past 15 years, the company has altered direction to develop bispecific antibody formats. It is also one of the first companies in the world to study the use of innate immunity for treating cancer.
These investigations have led to the development of the innate cell engager AFM13, a tetravalent, bispecific antibody molecule, a so-called TandAb. AFM13 is a protein consisting of a total of four antigen-binding domains, of which always two are identical. Two of these domains recognise the antigen CD30, a surface protein that frequently occurs on tumour cells of the lymphatic tissue, for example in Hodgkin's lymphoma or some T cell lymphomas. The other two domains bind to CD16A, an activating receptor on the surface of, for example, NK cells, which triggers the release of cytotoxic proteins upon binding. AFM13 therefore enables NK cells to specifically identify and destroy tumours.
Since 2015, the innate cell engager AFM13 has been tested worldwide in numerous phase II studies on patients that were no longer responding to conventional therapy. The targeted stimulation of NK cells has so far shown a favourable safety profile and is well tolerated. Neither the tumour lysis syndrome nor the cytokine storm frequently observed during the activation of T cells, both of which can lead to life-threatening organ failure, has occurred.
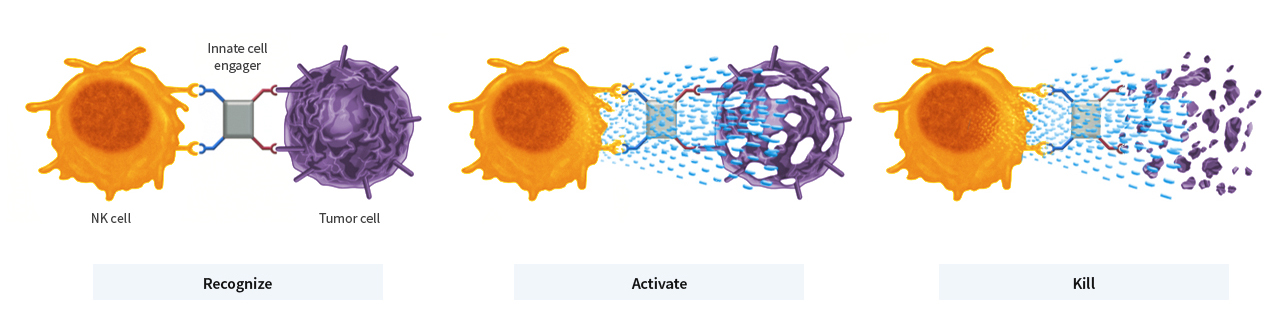 Innate cell engagers bring Nk cells or macrophages together with tumour cells, thus stimulating the immune cells’ cytotoxic activity, which leads to the destruction of the tumour cells. © Affimed GmbH
Innate cell engagers bring Nk cells or macrophages together with tumour cells, thus stimulating the immune cells’ cytotoxic activity, which leads to the destruction of the tumour cells. © Affimed GmbHAFM13 monotherapy has shown signs of therapeutic efficacy in CD30-positive T cell lymphomas: in 50 percent of the patients treated, AFM13 led to a partial or even complete remission of the tumour. AFM13 is currently being investigated as a monotherapy for treating CD-30-positive lymphomas in a phase II registration-directed trial.
The anti-tumour effect was less pronounced in patients with Hodgkin's lymphoma, which is why AFM13 is now being tested in a combination study involving AFM13 and the checkpoint inhibitor anti-PD-1. The addition of anti-PD-1, which prevents the adaptive immune response from being weakened by tumour cells, led to a response rate of almost 90 percent.
Affimed is also working with the MD Anderson Cancer Center (MDACC) in Texas to test AFM13 in combination with NK cells derived from healthy donors in CD30-positive lymphoma patients. In this novel approach, the role of the NK cells is to support the patient’s innate immune system, which has been weakened by previous chemotherapies. Preclinical data have shown the approach to be extremely promising and a phase I trial is in preparation.
The MDACC has also shown that administering genetically modified CAR-NK cells that have on their surface receptors for tumour antigens on B cell lymphomas is very successful and well tolerated1.
"Rather than using CAR-NK cells, we use non-manipulated and highly active NK cells, to which we add our innate cell engager. The latter binds to the NK cells, which produces a cell loaded with AFM13. The most important feature of our innate cell engager is its high binding affinity. This is a prerequisite for pre-loading," says Adi Hoess, CEO of Affimed since 2011, explaining his company’s strategy.
Innate cell engagers also affect the adaptive immune response
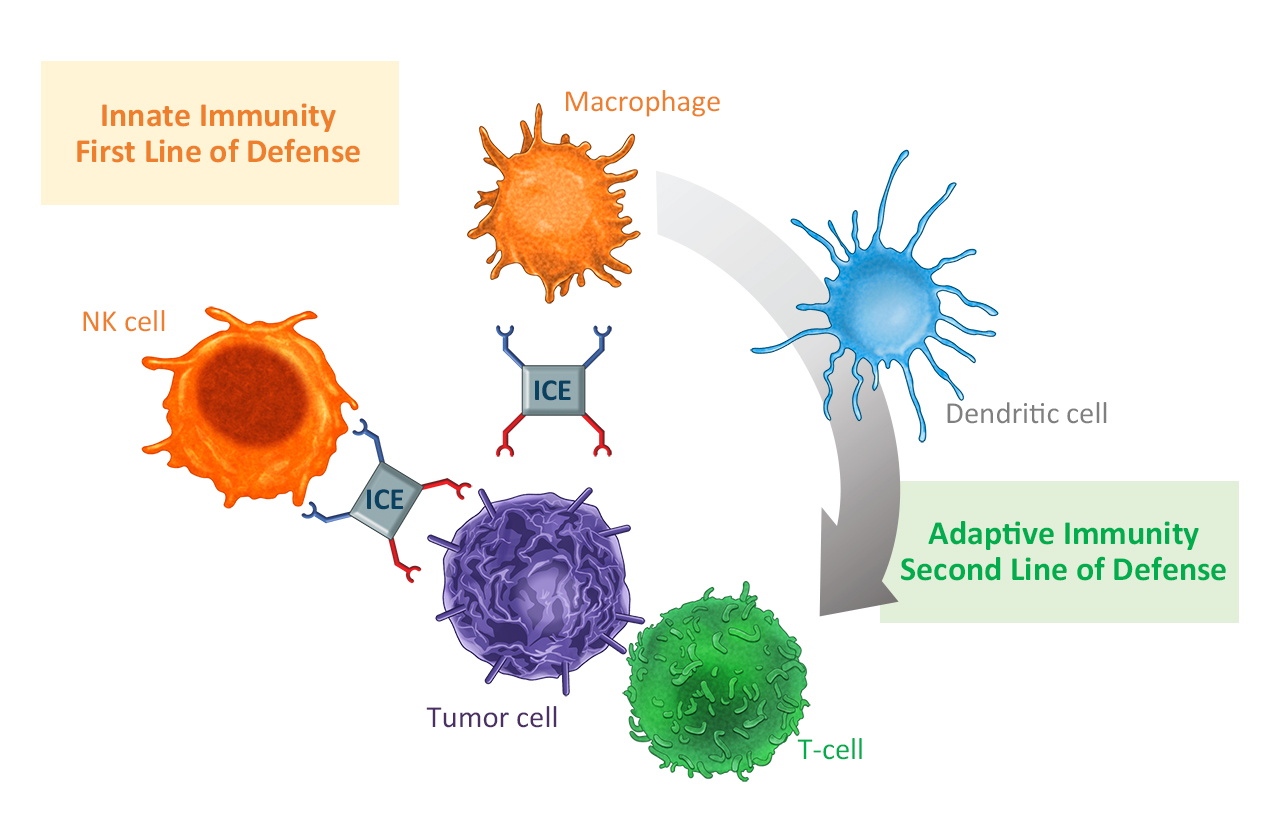 Communication between innate and adaptive immune defence. Innate cell engagers (ICE) bring together tumour cells with cells of the innate immune system, which in turn stimulate tumour-specific T cells. © Affimed GmbH
Communication between innate and adaptive immune defence. Innate cell engagers (ICE) bring together tumour cells with cells of the innate immune system, which in turn stimulate tumour-specific T cells. © Affimed GmbHThe CD16A receptor, which is bound by the innate cell engager, is not only located on NK cells but also on macrophages, which are then also recruited and activated. In addition to directly eliminating the tumour cells, the cells of the innate immune system therefore also stimulate the adaptive immune response.
"By activating both components of the immune system, we hope to significantly improve the patient’s chances of recovery. This is the big goal we are pursuing: we want to enable patients to eliminate the tumour using their own immune systems," explains Hoess, under whose leadership Affimed has emerged into a drug discovery and development company.
The ROCK® (redirected optimized cell killing) platform
To avoid having to construct every bispecific antibody molecule from scratch, Affimed has developed a platform called the ROCK® platform, which contains a large number of different antibody formats2. In addition to TandAbs, the platform can be used to produce larger, IgG-based molecules with longer half-lives, which can be advantageous for therapy. By exchanging the tumour antigen-binding domain, new candidates can be created within a few months. 90 percent of the construct remains unchanged, ensuring that all molecules are of the same high quality.
AFM24 is an IgG-based bispecific antibody molecule that is currently undergoing clinical phase I testing. AFM24 is directed against the EGF receptor, which is upregulated on many tumours. However, since this receptor is also widespread in healthy tissue, the currently used therapeutic approaches have strong side effects. In contrast, cells of the innate immune system only attack if they receive other activating signals from the tumour cell in addition to the stimulation by the innate cell engager; so healthy cells will not be affected. So far, hardly any side effects have been observed in preclinical studies; if this is confirmed in humans, it would be a major breakthrough.
Stimulating the innate immune system with innate cell engagers is a promising new approach in cancer immunotherapy, which, due to its high effectiveness and tolerability, has the potential to overcome the limits of conventional therapies.