RHEACELL GmbH
Innovative stem cell therapy for chronic wounds
Non-healing, chronically inflamed wounds can be very painful and carry the risk of serious infections. The Heidelberg company RHEACELL has developed a unique drug based on ABCB5-positive mesenchymal stem cells that helps reprogramme the relevant immune cells and promote healing.
More than a million people in Germany suffer from chronic wounds, i.e. injuries to the skin that have failed to heal even after eight weeks.1) In most cases, the cause is an underlying disease that increases tissue sensitivity and impedes the healing process. Circulatory problems due to narrowed arteries, for example, reduce the supply of oxygen and nutrients, whereas chronic venous wounds (CVUs; chronic venous ulcers) are caused by a weakness of the venous valves, which causes blood to back up in the lower veins and push erythrocytes into the tissue. The iron released from the red blood pigment then causes inflammation. Another chronic wound known as diabetic foot occurs when long-term elevated blood sugar levels in diabetes mellitus damage the vessels and nerves, restricting blood circulation. A weakened immune system can also delay the healing process. "The non-healing wound is trapped in a chronic inflammation cycle," explains Dr. Christoph Ganss, founder and CEO of RHEACELL GmbH & Co. KG. A novel cell therapy developed by the Heidelberg-based company has the potential to break this cycle and restore the physiological function of the tissue.
ABCB5-positive stem cells induce anti-inflammatory macrophages
Inflammations are the immune system’s natural reaction to damaging stimuli. Macrophages, special scavenger cells that eliminate damaged tissue in addition to foreign substances and pathogens, play an important role in this process. After an injury, M1 macrophages migrate to the affected site and additionally secrete pro-inflammatory mediators such as interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α), which attract and activate further immune cells. This first phase of wound healing is for cleansing and can last up to three days. During the subsequent proliferation phase, so-called granulation tissue first develops, which provisionally covers the wound, and is then transformed into resistant scar tissue in the subsequent epithelialisation phase. With a normal healing process, an acute wound closes within three weeks.
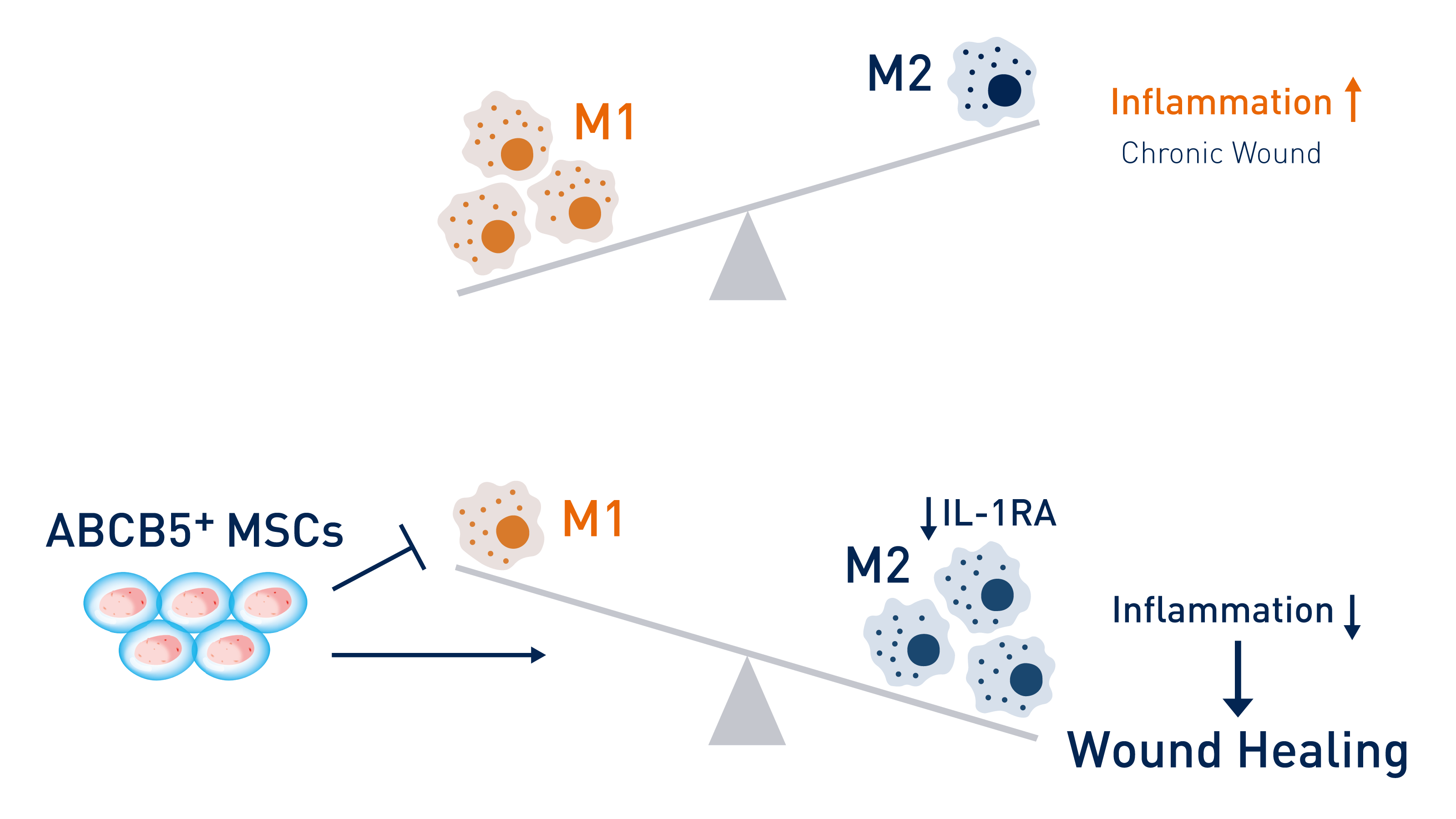 Chronic wounds contain mainly pro-inflammatory M1 macrophages that are trapped in a self-stimulating cycle. By releasing IL-1RA, ABCB5+ stem cells shift the balance to anti-inflammatory, pro-healing M2 macrophages. © RHEACELL GmbH & Co. KG
Chronic wounds contain mainly pro-inflammatory M1 macrophages that are trapped in a self-stimulating cycle. By releasing IL-1RA, ABCB5+ stem cells shift the balance to anti-inflammatory, pro-healing M2 macrophages. © RHEACELL GmbH & Co. KGIn common with other types of immune cells, macrophages have IL-1β receptors on their surface, leading to the self-stimulation of active M1 macrophages. To exit the inflammatory phase, this circuit must be interrupted to allow the pro-inflammatory M1 macrophages to convert into anti-inflammatory M2 macrophages. Here, the interleukin-1 receptor antagonist (IL-1RA) plays an important role, blocking the effect of cytokine IL-1β by preventing its binding to the IL-1 receptor. If the quantity of IL-1RA in the body is too low, the wound stagnates at the chronic inflammation stage.
This is where RHEACELL comes in with its cell therapy based on ABCB5-positive mesenchymal stem cells (ABCB5+ MSCs). Mesenchymal stem cells are multipotent progenitor cells of connective and supporting tissue that can develop into bone, cartilage, fat and muscle cells, for example. One particular subpopulation carries the regulatory protein ABCB5 (ATP binding cassette subfamily-B member 5) on its surface and has unique immunomodulatory properties, including the release of IL-1RA.
"The blockade of IL-1β is a key anti-inflammatory mechanism that causes the switch from M1 to M2 macrophages. The chronic wound thus becomes an acute wound that can heal," Ganss explains. "After the ABCB5-positive stem cells are administered into a wound, they settle in the tissue, interact with the surrounding immune cells and thus cause reprogramming. Administration of the antagonist alone does not have this effect." The M2 macrophages secrete, among other things, anti-inflammatory interleukin-10 and vascular endothelial growth factor (VEGF), which stimulate the formation of new blood vessels.
Patented manufacturing process
 Dr. Christoph Ganss develops innovative stem cell therapeutics with his companies RHEACELL and TICEBA. © RHEACELL GmbH & Co. KG
Dr. Christoph Ganss develops innovative stem cell therapeutics with his companies RHEACELL and TICEBA. © RHEACELL GmbH & Co. KGThe ABCB5+ MSCs, which constitute about two to three percent of the total dermal cell population, are obtained from donor skin. TICEBA GmbH isolates and multiplies these cells with the help of an elaborate, patented process and delivers a highly pure product that consists of more than 90 percent ABCB5-positive cells. TICEBA GmbH was founded by Ganss in 2003 as a stem cell bank, and is now responsible for all the production and quality control as well as all efficacy tests for the therapeutic agent, which belongs to the class of advanced therapy medicinal products (ATMP). TICEBA is the only licensee worldwide for products based on the ABCB5 molecule, which was first described in the early 2000s by Prof. Dr. Markus Frank from the Harvard Stem Cell Institute. RHEACELL GmbH, founded in 2012 as a joint venture between TICEBA and Müller Holding, conducts the clinical trials.
Data so far show that ABCB5+ MSCs are highly successful in treating CVUs.2) The locally administered stem cells are well tolerated and result in a wound size reduction of over 80 percent within twelve weeks in three out of four patients who have undergone the treatment. RHEACELL received a national marketing authorisation for its cell therapy AMESANAR® from the Paul Ehrlich Institute in October 2021, which means patients can benefit from the preparation before general approval is obtained. This allows specifically defined specialist groups to use the product in CVU patients. People with diabetic foot wounds also benefit greatly from the ABCB5+ MSCs, as ongoing studies show.
Also effective for genetic diseases
In addition to the application in chronic wounds, RHEACELL is also investigating the effect of stem cells in congenital and currently incurable severe skin diseases such as recessive dystrophic epidermolysis bullosa (RDEB). This is also known as butterfly disease because the skin of those affected is as fragile as the wings of a butterfly. Due to a genetic defect in the structural protein collagen VII, blisters can form anywhere on the body, including internal mucous membranes, and this results in open wounds. As there is no curative therapy yet, there are high hopes for RHEACELL’s pivotal trial starting in Europe and the USA in summer 2022.
RDEB is a systemic disease. Therefore, the cells are not applied to the wounds, but administered as an infusion. In cooperation with renowned international research groups, it has been shown in the mouse model that the ABCB5+ MSCs settle in the wounds (homing) and produce collagen VII as well as the structural proteins laminin and keratin 14 in addition to IL-1RA. Laminin and keratin 14 are deposited in the wound and thus enable healing. "Our cells have great homing potential and are therefore the ideal drug," says Ganss. "There are no rejection reactions at all because the MSCs are immune privileged and are not recognised as foreign. For this reason, we decided early on to work with allogeneic - i.e. foreign to the body - cells." These can be produced in advance and frozen so that they are quickly available when needed. Depending on the size of the wound, each patient then receives an individually adapted medication.
In the future, RHEACELL intends to expand the field of application of ABCB5+ MSCs to other diseases that are also based on a misdirected inflammatory response of the immune system, such as acute graft-versus-host disease.