Inside the fight against COVID-19
A new immunotherapeutic agent for treating severe COVID-19 cases
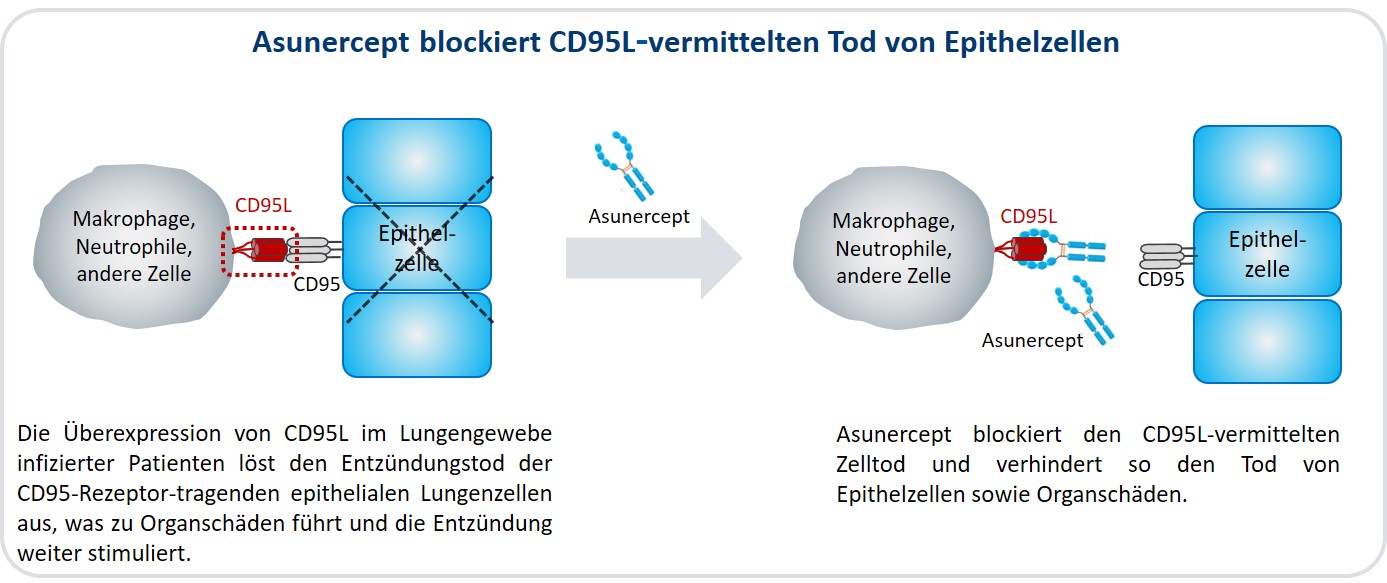
Apogenix, a Heidelberg-based biopharmaceutical company specialising in innovative immunotherapeutics, has initiated a Phase II clinical trial with asunercept, the company’s lead drug candidate for treating severe cases of COVID-19. The fusion protein blocks the CD95-ligand-mediated death of epithelial cells in the lung and thus prevents damage to the organ.
Without doubt, the COVID-19 pandemic is the worst health crisis of our time. From early this year, when the novel coronavirus was first reported in humans in Wuhan, Central China, until September 2020, well over 25 million people worldwide have been reported to be infected with SARS-CoV-2, and more than 850,000 have died from the COVID-19 disease caused by the virus (data from CSSE, Johns Hopkins University in Baltimore, USA). Since then, the number of people infected with SARS-CoV-2 has risen by around 250,000 a day and between six and eight thousand coronavirus-related deaths are reported daily. This corresponds to almost the total number of cases registered in Germany since the beginning of the pandemic.
Dysregulation of the immune system
As the name of the virus (SARS = severe acute respiratory syndrome) suggests, severe COVID-19 is characterised by an acute shortness of breath, which is accompanied by pneumonia and massive damage to the lung epithelium. In addition, the disease is associated with lymphopenia, a greatly reduced number of lymphocytes in the blood, especially in the early stages. As studies in Chinese COVID-19 patients have shown, there is a correlation between the severity of lymphopenia and disease severity and outcome.
Lymphopenia and pneumonia, which manifest themselves in acute respiratory distress syndrome (ARDS), are related to a misregulation of the immune system. A central role is played by the CD95 ligand (CD95L), whose natural function is to maintain immune homoeostasis by inducing cell death in reactive T lymphocytes. Binding of CD95L to the CD95 receptor (CD95) activates the cell's suicide programme (apoptosis). CD95L and CD95 are members of the superfamily of tumour necrosis factor and tumour necrosis factor receptor (TNF/TNFR), which play an important role in regulating the immune system and in the immune defense against tumours.
In viral diseases such as COVID-19 - as well as in SARS, MERS and influenza - the overproduction of CD95L induces severe malregulation of the immune system. The overexpression of CD95L has already been demonstrated in the lung tissue of infected patients (for example on inflammatory cells such as neutrophils and macrophages). The resulting excessive CD95L-mediated apoptosis of immune and epithelial cells leads to dysregulation of the immune system and triggers inflammatory destruction processes in the lung. This leads to pneumonia and ARDS.
Apogenix and asunercept
 Asunercept prevents the CD95L-mediated apoptosis of epithelial cells. © Apogenix
Asunercept prevents the CD95L-mediated apoptosis of epithelial cells. © ApogenixSince its foundation in 2005, Apogenix AG, a biopharmaceutical company based in Heidelberg that specialises in immunotherapeutic protein-based drugs, has built up a promising portfolio of drug candidates for treating cancer and viral infections. The effect of Apogenix’ drug candidates is based on their interference with different signalling pathways that depend on components of the TNF/TNFR superfamily. Apogenix' most advanced product is the CD95L inhibitor called asunercept, a soluble, fully human fusion protein composed of the extracellular domain of CD95 and the Fc part of the immunoglobulin G1.
Asunercept binds to CD95L, and prevents the activation of CD95, thereby impairing the signalling chains that are dependent on this receptor. It can thus prevent the apoptotic death of immune and epithelial cells. Asunercept has been developed for treating solid tumours and malignant haematological diseases and has orphan drug status in the European Union and the USA for treating glioblastomas and myelodysplastic syndromes (MDS). MDS is a heterogeneous group of malignant diseases of the bone marrow; glioblastomas are highly malignant brain tumours, which are particularly feared for their aggressive growth and high tendency to recur after removal of the primary tumour. The European Medicines Agency has granted asunercept "PRIority MEdicine" status (PRIME status) for the treatment of glioblastoma. Asunercept has also been shown to reduce lymphopenia associated with the overexpression of CD95L, as well as excessive inflammatory cell death of lung epithelial cells by blocking the ligand in patients experiencing ARDS in severe COVID-19 disease. Asunercept also has the potential to reduce lymphopenia associated with the overexpression of CD95L, as well as excessive inflammatory cell death of lung epithelial cells by blocking the ligand. The treatment of viral infections such as COVID-19 with asunercept represents an innovative therapeutic approach that directly targets two critical disease-causing mechanisms. It is now being tested in a clinical trial.
ASUNCTIS trial
 Dr. Thomas Höger, Chief Executive Officer of Apogenix AG. © Apogenix
Dr. Thomas Höger, Chief Executive Officer of Apogenix AG. © ApogenixIn July 2020, Apogenix announced that the company had received approval to initiate a Phase II clinical trial to assess the efficacy and safety of asunercept in patients with severe COVID-19. This ASUNCTIS trial is an open-label, randomised, controlled, multicentre trial involving 400 patients in Russia and Spain. The patients will be divided equally into four treatment arms: three groups of patients will be treated with three different doses of asunercept, while the fourth group will not receive the drug. The primary endpoint, i.e. the major objective of the trial, is increasing the time to sustained clinical improvement by at least one category on two consecutive days compared to the status at randomization. This will be measured on the clinical performance scale established by the World Health Organisation that covers the period from admission to hospital to day 29.
Secondary, i.e. efficacy endpoints of the ASUNCTIS trial include - in addition to the assessment of patients according to the "National Early Warning Score" – oxygenation requirement, mechanical ventilation requirement, duration of hospitalization including length of stay in an ICU, percentage of patients admitted to ICU and mortality on days 15 and 29. Thomas Höger, Ph.D., Chief Executive Officer of Apogenix AG, emphasised that excellent safety and tolerability and a clear trend towards the efficacy of asunercept has already been demonstrated in clinical trials involving patients with recurrent glioblastoma and myelodysplastic syndromes. Commenting on the ASUNCTIS study, he said: "We are very pleased to have obtained approval from the regulatory authorities in Russia and Spain to initiate our first clinical trial with our lead immunotherapy candidate asunercept in patients with severe COVID-19 and are confident that asunercept will prove to be effective in treating COVID-19 patients.”"