Immune complexes in COVID-19
Vicious circle of hyperinflammation
Whether a person experiences only a mild malaise or a critical course of disease after a coronavirus infection apparently depends on the antigen-antibody complexes that form in our body. The discovery of these immune complexes set a group of researchers, headed up by Prof. Dr. Hartmut Hengel from the Freiburg University Medical Centre, on the trail of a vicious circle of constantly escalating inflammation. The immune system is driven out of control, leading to a critical progression of the disease. This finding offers a promising basis for potential therapies.
In many cases, coronavirus infection causes only a minor cough and fever, but in some people it affects the whole body, necessitating mechanical and even extracorporeal respiratory support. There is growing clinical and experimental evidence that the body's own molecules are responsible for this. Virologist Prof. Dr. Hartmut Hengel and his team from the Freiburg University Medical Centre have found out what makes coronavirus infections, which primarily appear in the respiratory tract, so dangerous and explains why people suddenly develop multiple organ failure. The severe course of the disease is not caused by the virus itself, but by the body's own defences. The immune system, which is supposed to protect the body, reacts so strongly that the blessing of a strong defence system becomes a curse. "The immune response can be described as a concert with many instruments and different melodies," says Hengel. "In addition to T cells, messenger substances and specific immune cells, antibodies are just some of the many cells involved in immune responses."
Immune complexes identified as major players
 Prof. Dr. Hartmut Hengel and his team have elucidated the mechanism responsible for the severe course of COVID-19. © Prof. Dr. Hartmut Hengel, Freiburg University Medical Centre
Prof. Dr. Hartmut Hengel and his team have elucidated the mechanism responsible for the severe course of COVID-19. © Prof. Dr. Hartmut Hengel, Freiburg University Medical CentreThe researchers, led by Hengel, searched for the causative agents of the immune system overreaction and came across large complexes of antigens and antibodies that occurred almost without exception in patients with severe COVID. Surprisingly, these immune complexes contained neither viral material nor virus-specific antibodies. "We don’t think this has much to do with the virus itself," explains the virologist. Only the body's own molecules (self-antigens) and antibodies directed against them (autoantibodies) appear to be part of these aggregates. "And these immune complexes then lead to the immunological self-destruction of the body." In the past, their occurrence has often been associated with severe disease progression in autoimmune diseases, such as systemic lupus erythematosus (SLE) or rheumatoid arthritis.
The fact that the brain and kidneys are affected in critical cases supported the researchers’ assumption, and they found that this happens despite the fact that these organs contain little viral material. Unlike tissue-bound antigen-antibody complexes, it is in particular soluble immune complexes that circulate freely in the bloodstream that are responsible for spreading the inflammation throughout the body. Early detection of these complexes after clinical deterioration could be an indicator of the need for immediate anti-inflammatory therapy.
Overreaction due to autoimmunity?
 Team led by Prof. Dr. Hartmut Hengel: Dr. Philipp Kolb, Dr. Valeria Falcone, Dr. Sebastian Giese from the Freiburg University Medical Centre (from left to right). © Prof. Dr. Hartmut Hengel, Freiburg University Medical Centre
Team led by Prof. Dr. Hartmut Hengel: Dr. Philipp Kolb, Dr. Valeria Falcone, Dr. Sebastian Giese from the Freiburg University Medical Centre (from left to right). © Prof. Dr. Hartmut Hengel, Freiburg University Medical CentreIf the overreaction of the immune system, which results in hyperinflammation, could be attributed to the formation of autoantibodies directed against the patient's own body, this would clearly be an autoimmune reaction. The researchers suspect that this is what happens in patients with severe COVID, but the whole truth is yet to be discovered.
There are also other mechanisms of overreaction, such as the expression of activating receptors on immune cells where they are not normally found. Moreover, an altered sugar molecule signature on antibodies that are directed against the virus may result in a higher reactivity of the affected antibodies when the latter are recognised by immune cells. Both mechanisms lead to hyperinflammation and have been found in infected individuals with a severe disease course: due to the altered sugar signature, the virus-specific antibodies have too strong an effector function and bind to receptors located on the wrong cells. Moreover, soluble immune complexes enhance the normal immune response. "We believe that these two things are related, and that one overreaction somehow enhances the other," says Hengel, adding, "but we do not yet know which of the two comes first."
Virus triggers autoantibody production
Antibodies against the coronavirus are formed at an early stage as the body tries to fight off the viral infection. However, the altered sugar signature on the antibodies leads to an unusually strong immune response. "In severe COVID courses, these virus-specific antibodies do this in a very extreme way, resulting in massive tissue damage," Hengel points out. "The virus-specific antibodies produce an inflammatory environment and destroy virus-infected cells from which the body’s own molecules are released." Immune complexes are thus formed from virus antigens and virus-specific antibodies. Normally, macrophages break down these immune complexes by phagocytosis. However, if the phagocytosis system is overwhelmed, the complexes are deposited in the tissues, which calls other immune cells into action. This can lead to local inflammation, for example in small blood vessels, resulting in the release of self-antigens and inflammatory mediators such as cytokines and chemokines.
These signalling molecules stimulate the immune cells of the adaptive immune system, which in turn react highly sensitively to the new soluble immune complexes that have formed from self-antigens and autoantibodies. A receptor of the Fc receptor family suddenly appears on specific immune cells, i.e. T cells. Fc receptors are normally expressed on innate immune effector cells. The Fc receptor binds immune complexes and, when present on macrophages (which are innate immune effector cells), normally initiates the degradation of these complexes. However, when the Fc receptor is expressed in its active form as a signal transducer on T cells, which belong to the adaptive immune system, it gives the fatal command to produce inflammatory mediators and more autoantibodies.
As a result, the T cells experience an additional activation that overrides their normal tolerance not to attack the body’s own cells. "It is always quite dangerous when many immune cells are activated in one fell swoop and no longer follow their original command," says the researcher. "Suddenly, all T cells are controlled by this Fc receptor and produce cytokines, sparking uncontrolled inflammation." The resulting systemic tissue damage is not only maintained but exacerbated by the soluble and circulating immune complexes in the blood.
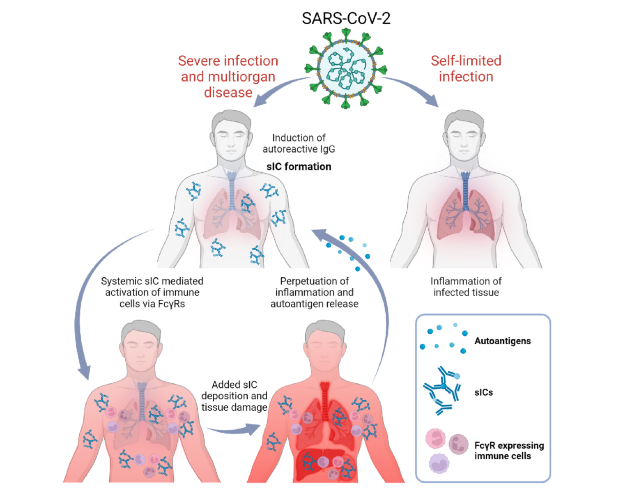 Depending on predisposition and autoantibody formation, COVID-19 can lead to severe systemic or only local infection. Source: Springer Nature I Philipp Kolb et al. I CC BY 4.0 https://creativecommons.org/licenses/by/4.0/
Depending on predisposition and autoantibody formation, COVID-19 can lead to severe systemic or only local infection. Source: Springer Nature I Philipp Kolb et al. I CC BY 4.0 https://creativecommons.org/licenses/by/4.0/
Predisposition as an additional factor
The vicious circle of overreaction is further driven by another mechanism. The highly activated T cells now cause the B cells to produce more autoantibodies that no longer have anything to do with the viral infection itself. Some patients have a predisposition to a low level of autoantibodies, which is harmless in itself. However, when the B cells are overstimulated, they produce more and more of these antibodies, which in turn are found in the immune complexes. Age is the major risk factor for immunologic derailment, because the immune system changes with age and appears to become more prone to error as a result of immune senescence. The action of immune complexes explains the systemic component of the disease well and is very similar in different autoimmune diseases.
The researchers did not find such immune complexes in patients with mild disease courses and immediately after vaccination. Therefore, they believe that the soluble immune complexes are an excellent biomarker for severe disease courses. It seems obvious that COVID treatment should involve the removal of the immune complexes and the autoantibodies from the patients’ plasma. This would be possible with an extracorporeal therapy, i.e. plasmapheresis, which is complex and expensive. "Plasmapheresis involves removing blood and circulating it through a machine where the plasma is separated, replaced by albumin and fluid, and returned to the patient," Hengel explains. However, the researchers are concerned that autoantibody formation will persist in the body and that the procedure will have to be repeated several times. Currently, treatment with cortisone and other anti-inflammatory agents is one of the most successful immediate therapies.