All-enzyme hydrogels in action
Biocatalytic foams enable the sustainable synthesis of complex molecules
Conventional chemical synthesis processes consume large amounts of energy and environmentally harmful solvents. Prof. Dr. Christof Niemeyer’s team at the Karlsruhe Institute of Technology has generated porous, solid foams from crosslinked enzymes that allow the production of high-quality compounds under significantly more environmentally friendly conditions. The novel biocatalysts are also extremely resistant and have a long shelf life.
The majority of industrially produced pharmaceuticals and cosmetics, as well as plastics, detergents and fertilisers, are based on basic inorganic and organic chemicals. These include sulphuric acid, chlorine, sodium hydroxide or ammonia, as well as ethylene, propylene and methanol. Both the synthesis of these simple compounds from fossil raw materials, and their further processing, require a lot of energy as well as large quantities of solvents that are harmful to human health and the environment. In addition, metal-containing catalysts are often used to accelerate the chemical reactions or to promote the production of certain variants. Although the chemical industry in Germany reduced its greenhouse gas emissions by 51 percent between 1990 and 2018, the carbon footprint of the aforementioned processes is still very high.1) The production of basic chemicals alone releases around 37 million tonnes of CO2 equivalents every year, accounting for 19 percent of Germany’s total industrial emissions.
The use of platform chemicals, i.e. basic chemicals made from renewable raw materials, can save resources and reduce the carbon footprint. However, their share in the chemical industry is still very low (around 15% in 2017) as their production is associated with higher costs compared to chemicals derived from fossil fuels.2)
Environmentally friendly biocatalysis
 Dr. Kersten Rabe, doctoral student Julian Hertel and Prof. Dr. Christof Niemeyer have developed novel biocatalytic foams at the Institute for Biological Interfaces at KIT. © KIT
Dr. Kersten Rabe, doctoral student Julian Hertel and Prof. Dr. Christof Niemeyer have developed novel biocatalytic foams at the Institute for Biological Interfaces at KIT. © KITAlternatives must also be found for the energy-intensive and pollutant-rich further processing of the platform chemicals. This is where the research of Prof. Dr. Christof Niemeyer, Director of the Institute for Biological Interfaces (IBG-1) at the Karlsruhe Institute of Technology (KIT), comes in. "We want to use enzymes to make chemical synthesis processes more sustainable. The biological catalysts, i.e. the enzymes, are highly specific compounds and only yield particular isomers [structural variants of a molecule, editor’s note]. This is difficult to control using conventional methods, which is why many worthless by-products are created." However, more importantly, enzyme-catalysed reactions can take place in an aqueous environment and at low temperatures. This makes them cheaper and much more environmentally friendly.
Both chemical and biological catalysts enable or accelerate the conversion of substances by reducing the required amount of activation energy. Both emerge unchanged from the reaction. They are therefore only required in small quantities and remain effective until chemical equilibrium is reached. However, continuous processes are possible when the catalysts are bound to a carrier material and the reactants flow past it. Binding molecules to a carrier material poses a particular challenge, especially when enzymes are used, as any change in the three-dimensional structure of the enzyme during immobilisation can lead to a loss of catalytic activity.
All-enzyme hydrogels for efficient conversion
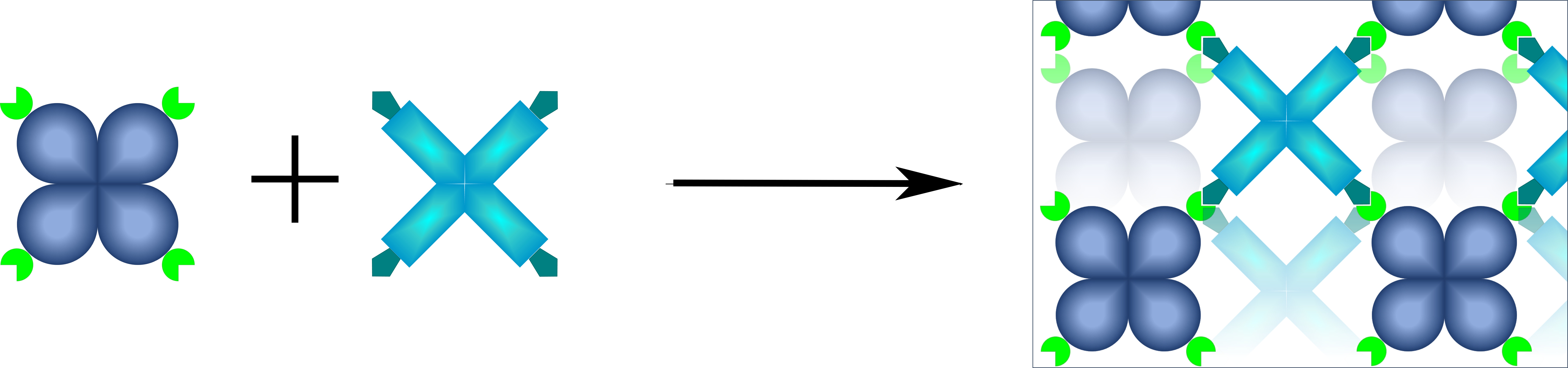 Under physiological conditions, the enzyme tetramers which are tagged differently, spontaneously form a uniform, porous network - an all-enzyme hydrogel. © KIT
Under physiological conditions, the enzyme tetramers which are tagged differently, spontaneously form a uniform, porous network - an all-enzyme hydrogel. © KIT"As much catalyst as possible needs to be present in a certain reactor volume in order to achieve high conversion rates," says Niemeyer, highlighting further requirements. "We have been working on this topic for quite some time and developed all-enzyme hydrogels five years ago." All-enzyme hydrogels are three-dimensional porous polymers that are made up of covalently cross-linked enzymes.3) Niemeyer’s team used two bacterial enzymes to synthesise specific alcohol isomers as well as to regenerate the necessary cofactor NADPH simultaneously. The enzymes used were an alcohol dehydrogenase (ADH) from Lactobacillus brevis and a glucose 1-dehydrogenase (GDH) from Bacillus subtilis.
Additional, but different, amino acid sequences, known as tags, were added to both proteins in the laboratory. When two of these dissimilar tags come together, they react spontaneously with each other and form a solid connection. As each enzyme is present with three others of its kind in a complex (homo-tetramer), it thus has four linkage sites. Mixing the homo-tetramers leads to the formation of a crosslinked porous enzyme polymer. Filling the all-enzyme hydrogel into the channel of a microfluidic chip that is subsequently perfused with a substrate solution leads to the almost complete conversion of the substrate solution into the desired product. "With this approach, we can achieve a high enzyme concentration as no additional carrier material is required. The hydrogels are also very resistant to the flow conditions in the reactor," says Dr. Kersten Rabe, highlighting the advantages of this approach. Rabe is a group leader in the Institute for Biological Interfaces 1 (IGB-1) in Karlsruhe focusing on biocatalysis and evolution and co-author of the publication describing the process.3)
Novel enzyme foams enable efficient flow-through biocatalysis
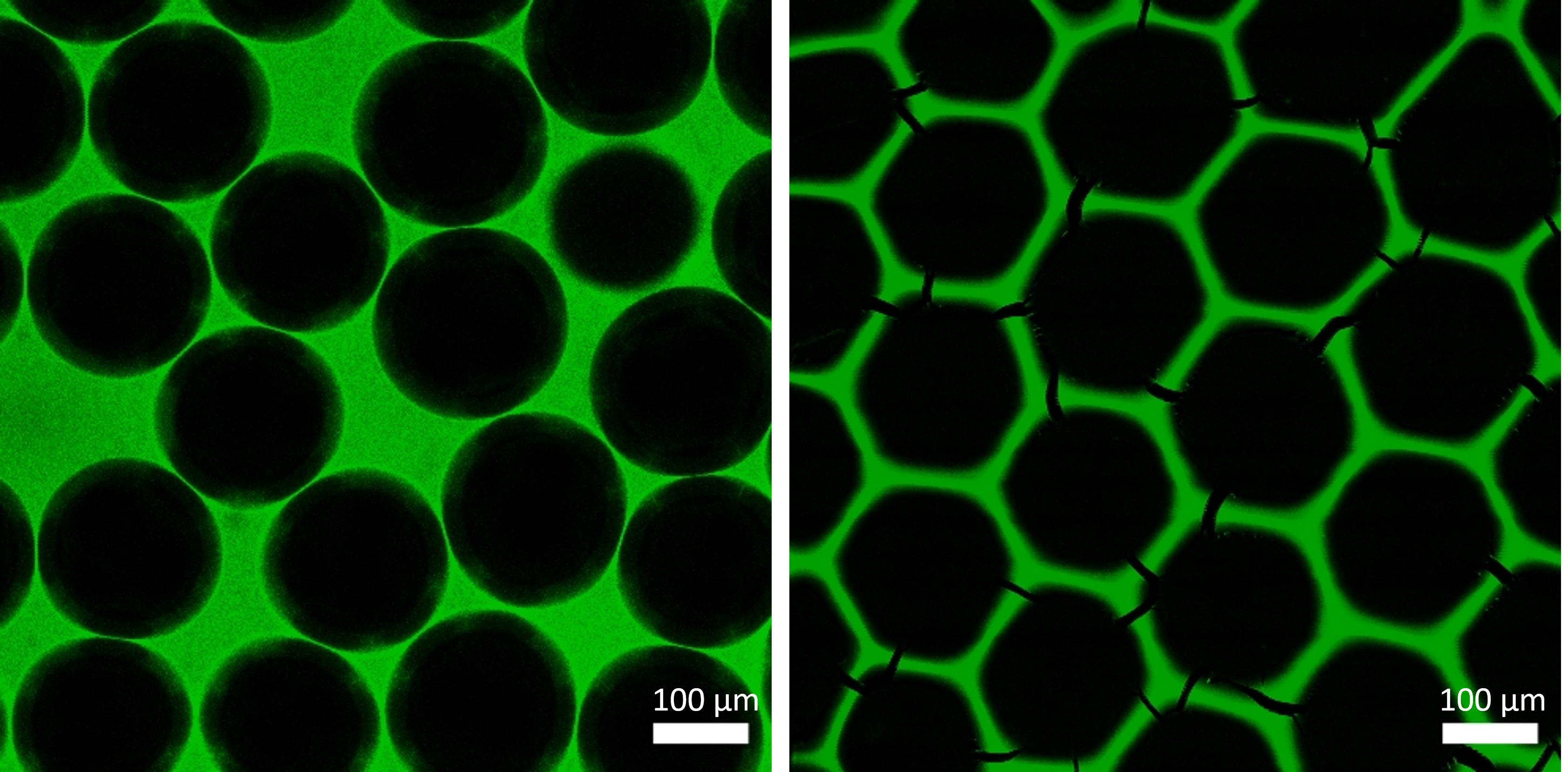 Foaming the all-enzyme hydrogel with nitrogen leads to the formation of air bubbles (left), which solidify upon drying in air (right) to form a regular, hexagonal lattice with a high surface-to-volume ratio (fluorescence microscope image). Source: doi:10.1002/adma.202303952, Julian S. Hertel | Karlsruhe Institute of Technology (KIT), CC-BY-NC-ND 4.0 (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.de)
Foaming the all-enzyme hydrogel with nitrogen leads to the formation of air bubbles (left), which solidify upon drying in air (right) to form a regular, hexagonal lattice with a high surface-to-volume ratio (fluorescence microscope image). Source: doi:10.1002/adma.202303952, Julian S. Hertel | Karlsruhe Institute of Technology (KIT), CC-BY-NC-ND 4.0 (https://creativecommons.org/licenses/by-nc-nd/4.0/deed.de)However, the enzyme polymer forms very small pores, which limits the transport of substances. In order to increase the flow rate and the surface-to-volume ratio, the researchers therefore foamed the gels in the chips with nitrogen. "Establishing the procedure was anything but easy, and a solution was needed as care had to be taken that the enzymes did not lose their three-dimensional structure," explains PhD student Julian Hertel, first author of the results published in the renowned journal Advanced Materials in September 2023.4) "But we now have the routines in place to reproducibly produce monodisperse [with bubbles of uniform size, editor’s note] foams."
The chips need to be dried for around four weeks before they can be used in the minireactor. This can be done easily at room temperature and in ambient air. The bubbles solidify during this process, forming a very regular hexagonal lattice. "The dried foams are incredibly stable, both physically and chemically. Some of the enzymes are even more active than in the fresh gels," says Niemeyer enthusiastically. So far, the researchers have successfully applied the process to two completely different enzyme systems. Niemeyer adds: "The uncomplicated storage, long shelf life and transferability to other enzyme classes is an important prerequisite for the future industrial utilisation of the process." A patent has already been applied for and the researchers are considering a spin-off.
The team is currently trying to further increase the stabilisation of the foams, as massive mechanical shear forces act when the liquid flows through the channels of the system. In addition, the process has already been transferred to proteins that are not present as tetramers.5) "We have a lot of experience in protein engineering," emphasises Rabe. "Adding two tags per enzyme enables the stable crosslinking of dimers. Higher degrees of multimerisation are also possible."
The use of innovative biocatalytic foams has the potential to make chemical synthesis processes more sustainable in the future. The researchers believe that the uncomplicated process, which has so far only been tested on a laboratory scale, will be easy to scale up. The concrete costs depend, above all, on the enzymes to be used, the production of which is, in most cases, still significantly more expensive than that of chemical catalysts. "As regards conventional methods, however, the costs of disposing of the environmentally harmful waste are often not taken into account," Niemeyer points out. Industry is already showing great interest in the new process which is particularly efficient when it comes to synthesising complex molecules.