Animal-free testing of chemicals and cosmetics
Human reporter skin for visualsing skin reactions
Before new cosmetics can be placed on the market, all ingredients must undergo rigorous testing for safety and efficacy. However, the European Union no longer permits the sale of cosmetic products tested on animals. Dr. Anke Burger-Kentischer of the Fraunhofer IGB in Stuttgart has long been at the forefront of animal-free research and has developed an innovative method to quickly and reliably test cosmetics and other chemicals without using animals. She was awarded the 2024 Hamburg Research Prize for the Promotion of the Development of Replacement and Supplementary Methods to Animal Testing for her groundbreaking ‘reporter skin’ in vitro model alongside the cosmetics company Beiersdorf.
Some substances simply don’t agree with our skin and can lead to tingling, itchiness or burning sensations. When a compound irritates skin cells, it can trigger a chain reaction of cellular signals that may lead to sensitisation or even inflammation. Some of these signalling pathways are already well understood scientifically and play a key role in applied research across various sectors, including the cosmetics industry. Since 2013, cosmetics tested on animals can no longer be sold in the EU. As a result, focus has increasingly shifted toward advanced human cell systems cultivated in the lab. These complex in vitro models allow researchers to assess the potential hazards of substances in a way that is directly relevant to human biology. This shift also addresses a long-standing challenge in biomedical research: translation failure - the limited ability of animal tests to accurately predict effects in humans.
Many new approach methods already authorised
 In the search for animal-free alternatives, Dr. Anke Burger-Kentischer at the Fraunhofer IGB in Stuttgart is developing innovative testing methods. One of the methods she has developed is called reporter skin, a groundbreaking in vitro model that enables real-time monitoring of skin reactions without the need for animal testing. © Dr. Anke Burger-Kentischer, IGB
In the search for animal-free alternatives, Dr. Anke Burger-Kentischer at the Fraunhofer IGB in Stuttgart is developing innovative testing methods. One of the methods she has developed is called reporter skin, a groundbreaking in vitro model that enables real-time monitoring of skin reactions without the need for animal testing. © Dr. Anke Burger-Kentischer, IGBNew approach methods (NAMs) are innovative technologies for health risk assessment that eliminate the need for animal testing. One such breakthrough is the living model developed and patented by Dr. Anke Burger-Kentischer and her team. Using real human skin samples, the model quickly, easily and cost-effectively reveals how skin cells respond to specific substances. Thanks to an integrated reporter system, it provides clear insights into the sensitising and pro-inflammatory potential of these substances.
Dr. Burger-Kentischer heads the Department of Cell and Tissue Technologies at the Fraunhofer Institute for Interfacial Engineering and Biotechnology (IGB) in Stuttgart, where she collaborates closely with the cosmetics company Beiersdorf. The probability that her innovation may soon reach the market reinforces her belief that she is on the right track with her research. NAMs that have previously been used to test skin-sensitising chemicals have come up against a number of limitations. Many relied on 2D cell cultures, which lack the complexity of multi-layered human skin. Testing oily or solid substances in conventional 3D models is also challenging – especially without a built-in reporter – since these are typically grown in aqueous nutrient solutions. Furthermore, subsequent analysis is labour-intensive, often requiring gene sequencing to determine which proteins are up- or down-regulated. If repeated exposure needs to be studied, multiple identical skin models must be used, as the process destroys the cells during analysis.
Reporter skin overcomes deficits
Dr. Burger-Kentischer has already developed many skin models and thought long and hard about analytics. "My motivation was to get away from animal testing and develop a simple and inexpensive read-out of the skin model," she says. "I always thought: there must be an easier way!"
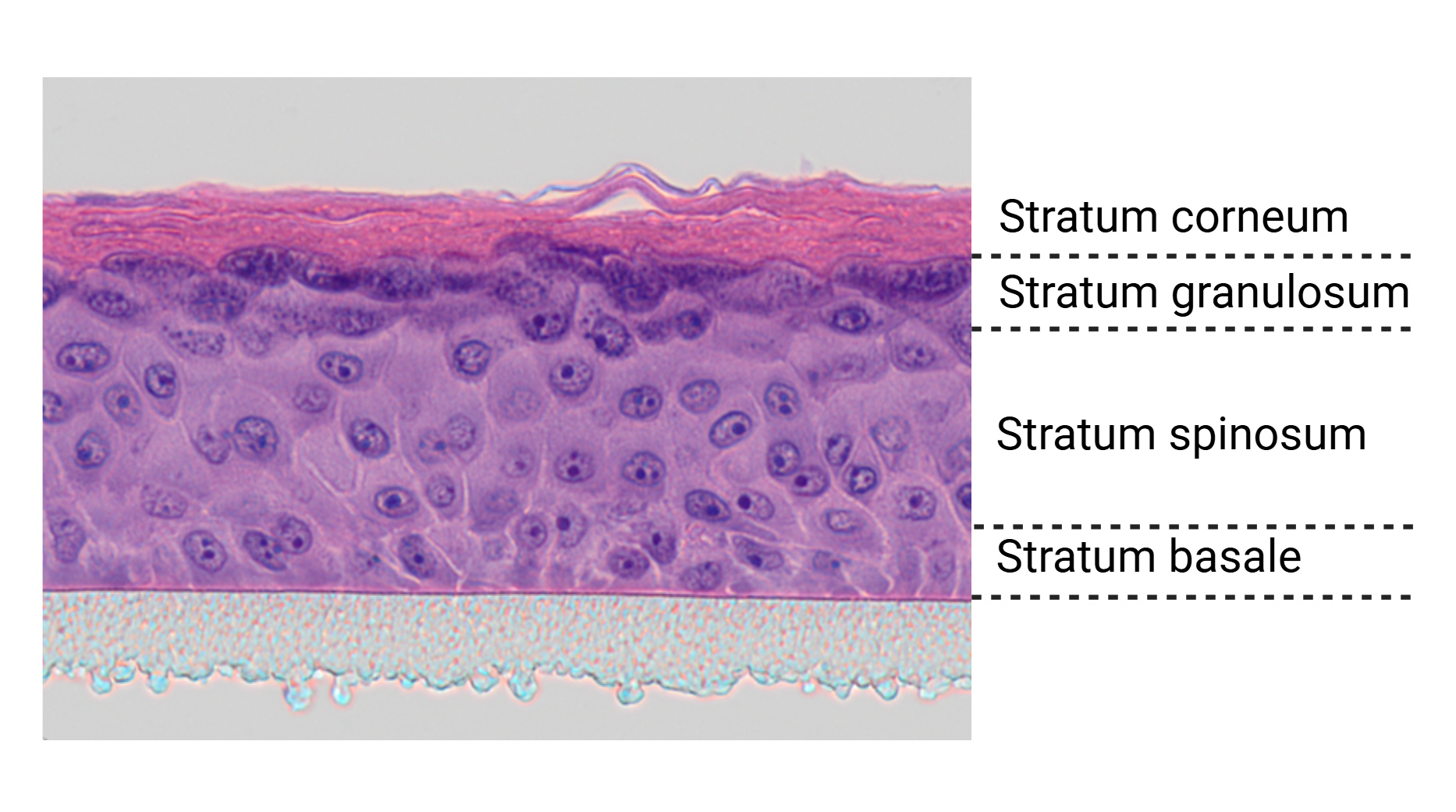 A microscopic cross-section of the three-dimensional in vitro reporter skin shows the epidermis (centre, pink), which is grown on a supporting membrane (bottom, grey) and develops a functional stratum corneum (top, pink) that acts as a barrier. The skin architecture of this 3D model closely mimics that of natural human skin, featuring distinct layers including the stratum corneum, stratum granulosum, stratum spinosum and stratum basale. © Fraunhofer IGB Stuttgart
A microscopic cross-section of the three-dimensional in vitro reporter skin shows the epidermis (centre, pink), which is grown on a supporting membrane (bottom, grey) and develops a functional stratum corneum (top, pink) that acts as a barrier. The skin architecture of this 3D model closely mimics that of natural human skin, featuring distinct layers including the stratum corneum, stratum granulosum, stratum spinosum and stratum basale. © Fraunhofer IGB StuttgartWith the development of her reporter skin model, Dr. Burger-Kentischer has introduced a physiologically relevant, organotypical system capable of detecting sensitising effects of chemicals, cosmetics and pharmaceuticals in preclinical testing – all without using animal models. In recognition of this advancement, she and her industry partner Beiersdorf were awarded the Hamburg Research Prize in 2024. The model, which enables the direct and reliable visualisation of immune-related skin responses, has also secured project funding from the International Collaboration on Cosmetics Safety (ICCS) – a global consortium of over 35 cosmetics companies and associations dedicated to promoting non-animal methods in product safety assessment.
Dr. Burger-Kentischer is also encouraged by the growing interest in NAMs within the chemical industry. She believes that the fully animal-free testing enabled by the reporter skin model – designed to closely replicate in vivo skin biology – has the potential to become a standard alternative method for large-scale assessment of skin stress. Skin stress can present as irritation, allergic reactions or inflammation. Depending on the type of external stimulus a skin cell encounters, different cellular stress signalling pathways are triggered.
Reporting on activated signalling pathway
The team at the IGB in Stuttgart developed the reporter skin to target the Nrf2 signalling pathway (Nrf2 stands for nuclear factor erythroid 2-related factor 2), which plays a key role in the skin’s response to sensitising substances. To determine whether a compound is a strong or weak sensitiser, Dr. Burger-Kentischer and her team ingeniously linked the activation of this pathway to the expression of a reporter gene. When the pathway is triggered, the resulting reporter protein is produced, thereby visibly signalling that sensitisation has occurred.
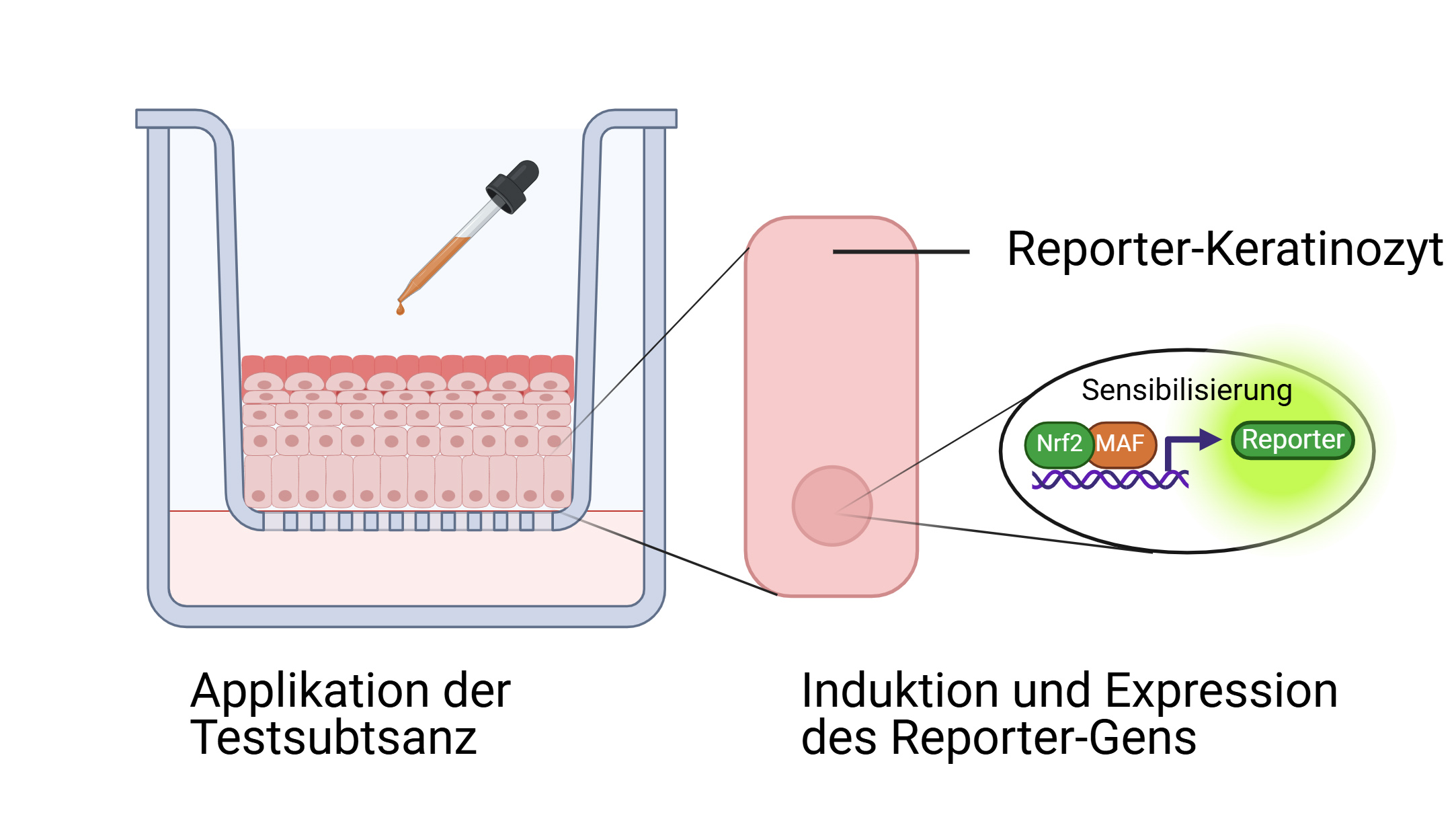 With reporter skin, cellular reactions become measurable through the activation of a signalling cascade that is linked to a reporter gene, resulting in a visible colour change. Test substances can be introduced not only through the culture medium but also applied directly to the surface of the epidermis (MAF: transcription factor). © Produced with BioRender.com
With reporter skin, cellular reactions become measurable through the activation of a signalling cascade that is linked to a reporter gene, resulting in a visible colour change. Test substances can be introduced not only through the culture medium but also applied directly to the surface of the epidermis (MAF: transcription factor). © Produced with BioRender.comDr. Burger-Kentischer sources healthy human skin samples from surgeries and clinics to create a special cell line with which reporter skin can be reproducibly regenerated under identical conditions. She isolates keratinocytes – the primary cells of the epidermis – from these samples that she then immortalises to make them permanently cultivable. Using a technique called nucleofection, in which a short electrical pulse delivers genetic material into cells, the reporter construct is introduced into the nucleus of these immortalised keratinocytes. "We then apply selection pressure to make sure the new DNA is stably integrated into the genome," she explains. The result is a cell line of immortalised keratinocytes carrying an inducible reporter gene, which is activated only when the skin’s sensitisation signalling pathway is triggered. The team has designed the system so that the reporter protein – an alkaline phosphatase – is secreted directly into the culture medium. When a substrate is added, the reporter protein produces a distinct yellow colour, indicating activation of the pathway. "Thanks to this pathway-specific expression," says Burger-Kentischer, "we can quickly and easily determine whether a substance has a sensitising effect – without the need for animal testing."
Reliable identification of skin stressors
The strength of the 3D reporter skin model lies in its strong physiological and structural resemblance to real human skin. It replicates the skin’s layered architecture, with keratinocytes forming the epidermis – including the protective barrier – and fibroblasts embedded in a collagen matrix forming the dermis underneath. Importantly, the cells retain the behaviour and marker expression typical of fully differentiated primary skin cells. The uppermost layer – the stratum corneum – remains dry, while the underlying fibroblasts deliver nutrients via the culture medium. This air-liquid interface enables oily or solid substances, such as textiles or creams, to be directly applied to the skin barrier, a feature that many alternative models lack. These properties make the system remarkably similar to in vivo conditions, delivering reliable and transferable results for human health risk assessment. Other advantages include rapid detection of cellular stress responses, high reproducibility, and the ability to apply substances repeatedly to the same model. The system also allows multiple time-point sampling, making it possible to track the dynamics of cellular responses over time. By incorporating different reporter genes, the model can simultaneously monitor distinct skin reactions – for example, by activating specific signalling pathways to determine whether a substance is pro-inflammatory, sensitising or allergenic.
The system was first validated using well-characterised reference substances, successfully demonstrating its functionality. The current project funding from the International Collaboration on Cosmetics Safety (ICCS) marks a second, more advanced stage of validation. Dr. Burger-Kentischer’s ultimate goal is to have reporter skin formally recognised as a regulatory method for assessing skin sensitisation risk. "As soon as it is included in OECD guidelines, companies will be able to use it for official safety testing," she explains. In the meantime, the Fraunhofer IGB offers a range of services related to substance analysis and the use of the reporter skin model. Looking ahead, the research team is already exploring new applications - from expanding the system for use in other types of tissue models to transferring the technology to entirely new contexts, such as "WowWowSkin – in vitro canine skin equivalents", a project that focuses on veterinary dermatological testing.