High-resolution microscopy technology bypassing the diffraction limit
From micro- to nanoscope
It has long been impossible to distinguish objects closer than 200 nanometres using light microscopes. However, novel devices developed by a company called abberior Instruments GmbH, which use technology developed by Nobel Prize winner Prof. Dr. Stefan Hell and his teams in Heidelberg and Göttingen, are now able to bypass this resolution limit and provide detailed insights into living cells in the lower nanometre range.
Human cells are 1 to 30 micrometres in diameter and cannot be discerned with the naked eye. The fine structures they contain are many times smaller: the diameter of membranes or individual proteins, for example, is in the range of a few nanometres (nm, 10-9 m). As early as the 17th century, the physician and biologist Jan Swammerdam used a microscope (from the Greek mikrós = small and skopeín = to look at) to examine animal and human tissue and body fluids. With the help of the approximately 200x magnification that was already possible at the time, he was the first to discover blood cells, red blood cells to be more precise.
The Abbe limit restricts light microscopy
 abberior Instruments revolutionises microscope technology. The company founders (from left to right): Dr. Lars Kastrup, Dr. Benjamin Harke, Dr. Andreas Schönle, Dr. Gerald Donnert, Prof. Dr. Stefan Hell, Dr. Matthias Reuss, Dr. Christian Wurm, Dr. Alexander Egner. © abberior
abberior Instruments revolutionises microscope technology. The company founders (from left to right): Dr. Lars Kastrup, Dr. Benjamin Harke, Dr. Andreas Schönle, Dr. Gerald Donnert, Prof. Dr. Stefan Hell, Dr. Matthias Reuss, Dr. Christian Wurm, Dr. Alexander Egner. © abberiorOver the next few centuries, light microscopy was increasingly refined by advances in optics, specimen preparation and illumination techniques. However, due to the wave nature of light, for a long time it was only possible to optically distinguish between objects that were at least 200 nm apart from each other. This corresponds to half the wavelength of visible light. This resolution limit, also known as the diffraction limit, was described by the German physicist Ernst Abbe as early as 1873. A significantly better resolution (of up to 0.1 nm) can only be achieved using electron microscopes, because the electron beams used in this case have a much shorter wavelength. However, because this technology requires samples to be anhydrous, electron microscopy cannot be used to examine living cells.
Until 25 years ago, the diffraction limit was considered insurmountable. Then, at the end of the last millennium, Prof. Dr. Stefan Hell of the Max Planck Institute (MPI) for Biophysical Chemistry in Göttingen (now MPI for Multidisciplinary Sciences) successfully developed a novel method that achieves a resolution of 20 - 30 nm in light microscopes. "We used a trick to make things that were previously hidden, i.e. below the diffraction limit, visible," explains physicist Dr. Matthias Reuss, who did his doctorate in Hell’s working group at the German Cancer Research Center (DKFZ) in Heidelberg from 2003 to 2017 and helped improve the technology. Together with six other scientists from Heidelberg and Göttingen, Hell and Reuss founded abberior Instruments GmbH in 2012 with the aim of making the new high-resolution microscopy technology accessible to people without extensive knowledge of physics.
STED technology bypasses diffraction limit and achieves higher resolution
Fluorescent substances have been used in light microscopy for decades to make individual components of biological samples easier to identify. When irradiated with light of a certain wavelength, these components can absorb a "light particle", a photon, and enter a higher-energy, excited state. The photon is emitted again when it returns to its ground state. However, it is then lower in energy, i.e. it has a longer wavelength, causing substances to fluoresce with a different colour. Some biological molecules, such as the green chlorophyll in plant cells or the collagen protein, are autofluorescent. But other molecules can also be specifically made to glow by linking them with fluorescent compounds called fluorophores, which then makes them easy to study.
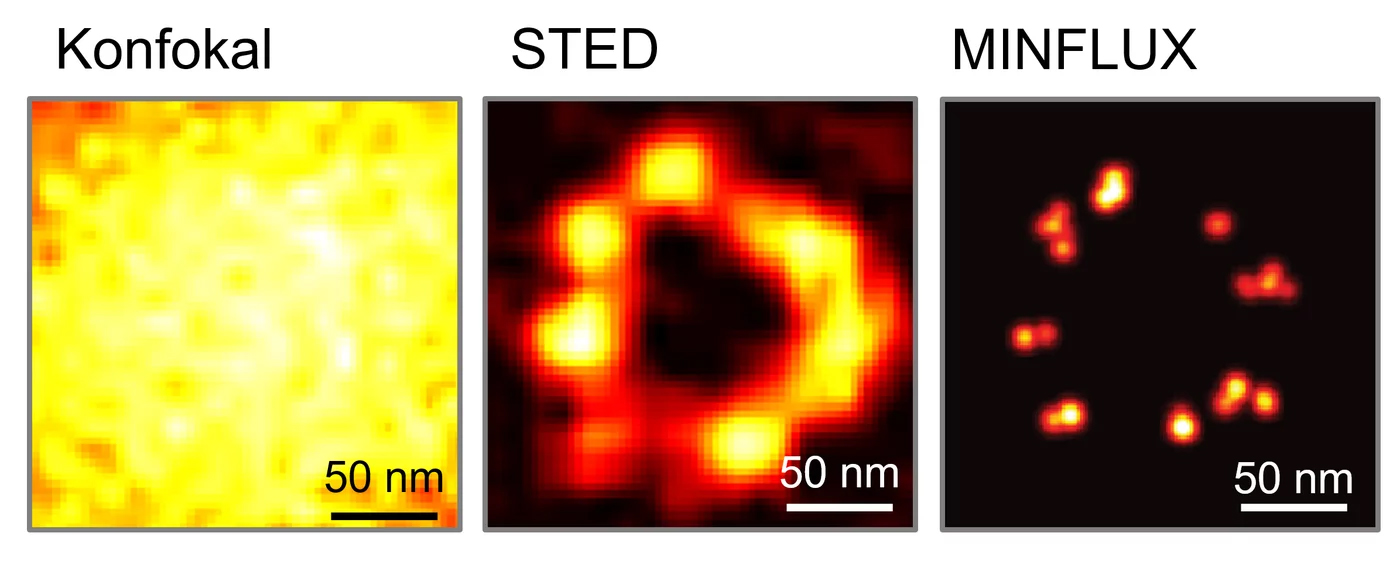 Comparison of light microscopy images of the protein NUP96, a component of the pore complex of cell nuclei: the individual NUP96 proteins cannot be distinguished from each other using classical confocal microscopy; they appear as a single light spot. STED microscopy increases the resolution by about 10x and can overcome the Abbe limit. MINFLUX significantly increases the resolution of an image (up to a few nanometres). © Working group Hell/MPI-NAT
Comparison of light microscopy images of the protein NUP96, a component of the pore complex of cell nuclei: the individual NUP96 proteins cannot be distinguished from each other using classical confocal microscopy; they appear as a single light spot. STED microscopy increases the resolution by about 10x and can overcome the Abbe limit. MINFLUX significantly increases the resolution of an image (up to a few nanometres). © Working group Hell/MPI-NATThe innovative technology developed by Hell, which bypasses the diffraction limit and thus increases the resolution, is a special variant of fluorescence microscopy. "The basic principle is to manipulate the fluorescence molecules rather than the light, so that the dyes of neighbouring objects do not fluoresce simultaneously and their signals do not overlap," explains Reuss. This is achieved by illuminating a sample with an excitation laser pulse followed by a depletion laser pulse, which prevents the molecules from emitting light. This STED (stimulated emission depletion) beam looks like a doughnut, i.e. it has a hole or a zero at its centre. As a result, only the molecules in the small central area fluoresce, while those at the edges of the beam remain dark. To obtain a complete image of the sample, it is then scanned point by point, as in a confocal microscope. The physicist explains: "This was the decisive trick: the beam itself is still diffraction-limited, but its effect on the molecules is not. The stronger the beam, the smaller the zero or hole becomes, and the higher the resolution." Theoretically, resolution does not have a limit, but in practice it is 10 to 20 nm. Otherwise, the number of photons required would be too high and the illuminated molecules would be destroyed or bleached out.
Hell was awarded the Nobel Prize in Chemistry in 2014 for the revolutionary concept of ultra-high resolution STED nanoscopy. He shared the prize with the Americans Eric Betzig and William E. Moerner, who achieved similar resolution by selectively illuminating individual fluorescently labelled molecules (PALM and STORM technology for photoactivated localisation microscopy and stochastic optical reconstruction microscopy, respectively).
Shoebox-sized nanoscope developed and manufactured in Heidelberg
 The shoebox-sized STEDYCON (image a) expands all conventional systems via a free camera access into a confocal microscope as well as a high-resolution STED nanoscope. MINFLUX (image b) is the world's most powerful light microscope. © abberior
The shoebox-sized STEDYCON (image a) expands all conventional systems via a free camera access into a confocal microscope as well as a high-resolution STED nanoscope. MINFLUX (image b) is the world's most powerful light microscope. © abberiorabberior Instruments now markets four different types of devices, which have so far been used mainly in academic biology and medical research. However, there is increasing interest in the devices from the pharmaceutical industry.
The FACILITY platform is a STED and confocal microscope that allows beginners to produce first-class images thanks to intuitive operation but also offers plenty of other possibilities for experts. The INFINITY platform caters for specific customer requirements and is tailored to special construction and small series.
The STEDYCON, developed and manufactured in Heidelberg, is very compact and attractively priced. It can be used to upgrade any existing microscope stand to a confocal microscope and STED nanoscope. It is robust, can be operated without prior knowledge and achieves a resolution of 30 nm.
MINFLUX is currently the world’s highest resolution light microscope. It can resolve biological structures as small as molecules with unprecedentedly high spatial (< 1 nm) and temporal (up to 10 kHz) resolution. This makes biological processes, such as changes in protein conformation, directly visible in the natural environment of living cells - at an investment cost comparable to that of conventional microscopes. And the technology still has the potential for further improvements.
A company firmly in the hands of researchers
The company history of abberior is unusual. "Eight of us started the company with no external financing. The proceeds from the first device were used to build others, and we slowly expanded our offer," says Reuss, describing the company’s approach. To this day, the company, which is headquartered in Göttingen, is run entirely by researchers. "This allows us to bring new technologies, such as the MINFLUX, to market quickly." The company currently employees 120 staff worldwide, with sales and support offices in the USA, Switzerland and China. All production takes place in Germany. Heidelberg, with approximately two dozen employees, is responsible for the STEDYCON; the other devices are manufactured in Göttingen, where the company’s administration is also located.
"We do a lot of research and development ourselves. Most of our employees are incredibly well-trained physicists, biologists or chemists." In addition, the company implements technologies developed by Hell's working groups at the MPI for Medical Research in Heidelberg and the MPI for Multidisciplinary Sciences in Göttingen and licensed out by the Max Planck Society. The name says it all: "We claim to build the best microscopes with the highest resolution that are also easy to use. The company name abberior is derived from "Ernst Abbe" and from the fact that we are "superior" - i.e. better than the diffraction limit that he formulated," says the head of research and development, Matthias Reuss, with a smile. In keeping with this, the company is the only microscope manufacturer in the world that also develops suitable fluorophores for all fluorescence technologies, so that instruments and dyes are perfectly matched.
Info box: How the MINFLUX microscope works
The technology is based on closely spaced molecules that are made to fluoresce one after the other with their position then being precisely determined. This is also done using a doughnut-shaped beam of light, but this is an excitation beam with a zero at its centre. This beam is shifted until the fluorescence is minimal because it is directly below the zero. Since its position is known, the position of the molecule is also known. The position of a fluorescence molecule is therefore determined by registering as few photons as possible rather than as many as possible, as is the case with conventional microscopy techniques. As a result, the efficiency of a MINFLUX microscope (measured as the number of photons required relative to the resolution achieved) is orders of magnitude better than that of a conventional microscope.