Breath test replaces invasive diagnostics
Novel smartphone sensor offers innovative breath test to detect Helicobacter infection
The stomach bacterium Helicobacter pylori is widespread and, if left undetected, can impact our well-being and also lead to serious health conditions. While effective treatments exist, a reliable diagnosis is essential. To address this, researchers at the University of Ulm have developed an affordable breath test that uses a mini-sensor to detect the presence of the bacterium. They are currently working on a smartphone-compatible version, with the aim of making it widely available for self-testing.
Take a tablet or an oral gel, wait 30 minutes, then simply blow into the test device equipped with a mini-sensor and receive a yes-or-no answer about a possible Helicobacter pylori infection. This can be done at home or in the doctor’s surgery and it is what diagnosis could soon look like.
However, the current diagnostic process is far more complex: if an infection with the bacterium is suspected, a gastroscopy is performed to obtain a biopsy of the gastric mucosa, which is then analysed in the lab for bacterial presence. A non-invasive alternative does exist - analysing the patient's breath using laboratory techniques such as mass spectrometry or non-dispersive infrared (NDIR) spectroscopy. However, this method is costly and relatively complex.
These are not occasional tests performed in specialised centres - they are carried out millions of times a year. That’s because Helicobacter pylori infects the stomach lining of over 50% of the global population, making it one of the most prevalent bacterial pathogens worldwide.1)
Survivalist Helicobacter bacteria - often symptom-free but not harmless
The bacterium is typically acquired in childhood and causes chronic inflammation of the stomach lining, known as gastritis. In many cases, it remains unnoticed - more than 80% of people infected are symptomless. However, over time this persistent inflammatory state can lead to serious secondary conditions, including stomach ulcers and even gastric cancer if left undetected for decades. The stomach’s highly acidic environment is generally hostile to bacteria, making Helicobacter pylori exceptional: it is the only known microorganism capable of surviving long-term in the human stomach.
So what sets this gastric pathogen apart from other microorganisms? Helicobacter pylori owes its remarkable acid resistance to a sophisticated survival mechanism. While most microbes are quickly destroyed by the stomach’s aggressive acid, Helicobacter forms a chemical shield by producing alkaline ammonia (NH₃), which neutralises the acidic environment in the human stomach. This is made possible by the enzyme urease, which the bacterium produces in large quantities. Urease uses water to catalyse the breakdown of urea into carbon dioxide (CO₂) and ammonia (NH3). Urea and water, the starting substances, are readily available in the stomach.
Miniaturised test harnesses exhaled CO2
 At the University of Ulm, Prof. Dr. Boris Mizaikoff is developing breath-based diagnostic systems that detect health-related biomarkers, which serve as unique 'fingerprints' for specific diseases. © Hahn-Schickard
At the University of Ulm, Prof. Dr. Boris Mizaikoff is developing breath-based diagnostic systems that detect health-related biomarkers, which serve as unique 'fingerprints' for specific diseases. © Hahn-SchickardThe breath test for Helicobacter pylori takes advantage of the bacterium’s unique chemical defence: "We focus on CO₂, the by-product of bacterial urease activity," explains Prof. Dr. Boris Mizaikoff, Head of the Institute of Analytical and Bioanalytical Chemistry at the University of Ulm and the Hahn-Schickard Institute in Ulm. Mizaikoff is a pioneer in breath diagnostics, who has been developing respiratory measurement systems for nearly 20 years. Since 2023, he has also been contributing to the EU project M3NIR (Integrated, Modular, Multisensing, Mid- and Near-IR sensing platform). This initiative brings together 13 international partners to create integrated, modular multi-sensor platforms for monitoring health and environmental parameters on a microscale. The results have been promising: Mizaikoff and his team have successfully miniaturised the Helicobacter breath test, making it portable, fast, cost-effective and ideal for widespread, high-throughput diagnostic use - without placing additional strain on the healthcare system.
A well-established method in medical diagnostics is used to ensure that the exhaled CO₂ really originates from Helicobacter pylori and not from the person’s normal respiration. This method is stable isotope labelling with carbon-13 (13C), a harmless variant of the naturally abundant 12C. In the test, 13C-labelled urea is administered as a powder or oral gel. If Helicobacter is present in the stomach, its urease enzyme breaks down the urea, releasing 13CO2, which is then exhaled and detected. 13C from the sample, if metabolised by the test subject rather than by Helicobacter, would be excreted through the gastrointestinal tract and would not interfere with the accuracy of the test.
Small, simple, affordable and accurate
 Side view of the mini-sensor with infrared-permeable barium fluoride windows, which ensure that the MIR radiation is reflected along the channel and the absorbed wavelengths of 13CO2 and 12CO2 can be measured. © Dr. Gabriela Flores Rangel/Ulm University
Side view of the mini-sensor with infrared-permeable barium fluoride windows, which ensure that the MIR radiation is reflected along the channel and the absorbed wavelengths of 13CO2 and 12CO2 can be measured. © Dr. Gabriela Flores Rangel/Ulm UniversityFor the test, the exhaled carbon dioxide is analysed to distinguish between 13CO2 - produced through bacterial activity - and the naturally occurring 12CO2 found in normal respiration. Mizaikoff and his team have developed a spectroscopic technique based on mid-infrared (MIR) light at a wavelength of approximately 4.3 µm. "This is essentially thermal radiation," Mizaikoff explains, "and 13CO2 absorbs it at a slightly lower, yet clearly distinguishable, wavelength than 12CO2. This allows us to detect whether 13CO2 is present in the exhaled air - an indicator of Helicobacter pylori infection. Moreover, by measuring the ratio of 13CO2 to 12CO2, they can quantify how much carbon originates from the bacterially cleaved urea. "The method is significantly more cost-effective and easier to handle than mass spectrometry and is also well-suited for miniaturisation," he adds.
The light absorption of the molecules is measured using a mini-sensor developed by Prof. Mizaikoff in collaboration with his postdocs, Dr. Gabriela Flores Rangel and Dr. Lorena Díaz de León Martínez. The technology is based on so-called substrate-integrated hollow waveguides (iHWG), which significantly reduce the reaction space to optimise the interaction between the sample molecules and the infrared radiation. 2) "Typically, when optical systems are miniaturised, the analytical quality of the signal deteriorates," explains Mizaikoff. "However, we’ve managed to downscale the sensor system without compromising measurement precision."
Goal: consumer test system as a smartphone add-on
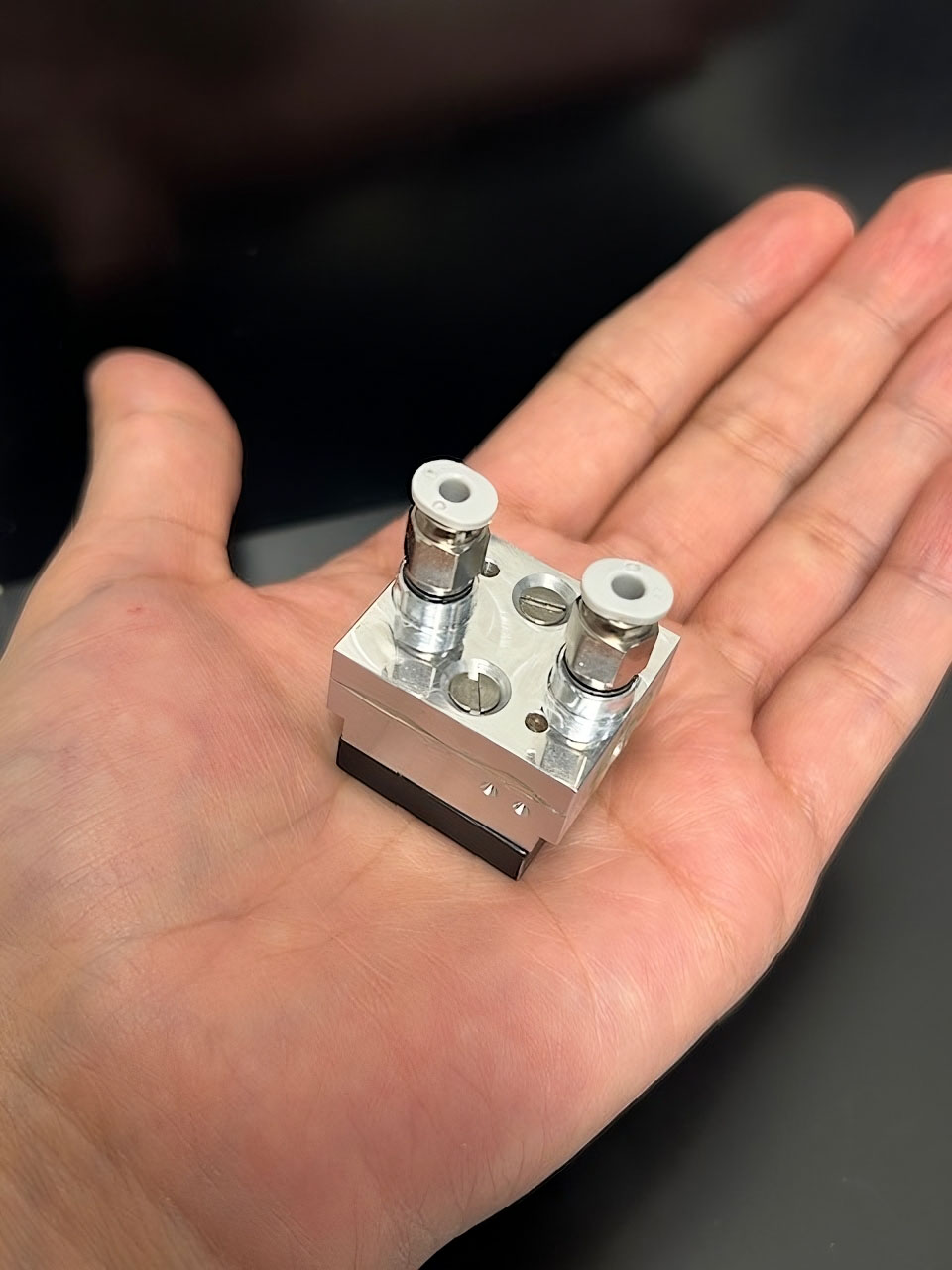 The miniature reaction chamber is composed of two aluminium plates, hermetically sealed with an epoxy resin compound. A narrow channel embedded in the base plate serves both as a gas cell and an optical waveguide, directing the exhaled breath through the chamber while guiding the infrared light for analysis. The next step is to further miniaturise the sensor to enable seamless integration with smartphones, paving the way for accessible, point-of-care diagnostics. © Dr. Gabriela Flores Rangel/Ulm University
The miniature reaction chamber is composed of two aluminium plates, hermetically sealed with an epoxy resin compound. A narrow channel embedded in the base plate serves both as a gas cell and an optical waveguide, directing the exhaled breath through the chamber while guiding the infrared light for analysis. The next step is to further miniaturise the sensor to enable seamless integration with smartphones, paving the way for accessible, point-of-care diagnostics. © Dr. Gabriela Flores Rangel/Ulm UniversityThe breath test with the innovative mini-sensor initially delivers a straightforward yes-or-no result regarding the presence of a Helicobacter pylori infection. "This result can then be followed up clinically - with targeted antibiotic therapy - or confirmed through more detailed diagnostics," explains chemist Prof. Dr. Boris Mizaikoff. "We’re also working on extending the system’s capabilities to allow quantification in the future." A smartphone-compatible add-on is in development for use in remote areas that have no medical infrastructure and for home testing. "With infrared LEDs, we envision a low-cost photonic consumer product priced at around 30 euros - something that could be sold in pharmacies or supermarkets for easy access when symptoms arise." An initial patent has already been filed. The current mobile-phone-sized prototype is expected to be finalised by the end of the year, with a significantly smaller version anticipated by mid-2026.
However, the Helicobacter test is not the only innovation to emerge from the Ulm researchers' laboratory. "The molecular signature in human breath is highly complex, yet characteristic and even deeply personal - essentially a unique fingerprint for certain diseases," says Prof. Dr. Boris Mizaikoff. "Helicobacter pylori was an ideal starting point for us because it is a pathogen that can be detected with a single molecular marker. It served as a compelling proof of concept for developing respiratory diagnostic systems that are compact, affordable and scalable," he adds. "More sophisticated applications - such as breath tests for COVID-19, gastric cancer or breast cancer - are already in development and currently undergoing preclinical validation through clinical partners in Mexico. These systems are still larger - roughly the size of two shoeboxes - but we're actively working on miniaturisation across various applications. Our broader goal is to make sensor technologies portable and bring analytical methods out of the lab and into everyday use. Breath diagnostics will be a key beneficiary of these advances."