Cancer cells possess properties that differ fundamentally from those of healthy cells. They do not require external growth factors to be able to proliferate indefinitely and they do not respond to growth-inhibiting signals. They are able to grow into foreign tissue and form metastases. Moreover, they can evade programmed cell death (apoptosis), which normally causes damaged cells to commit suicide, and can also promote the formation of new blood vessels (angiogenesis) in their environment, so that they can be supplied with the nutrients they need.
 Dr. Claus Kremoser is co-founder of WMT AG, which develops novel cancer therapies to prevent tumour cells from producing lactate. © Claus Kremoser
Dr. Claus Kremoser is co-founder of WMT AG, which develops novel cancer therapies to prevent tumour cells from producing lactate. © Claus KremoserMany cancer cells rewire their energy metabolism during the growth phase, which gives them a selective growth advantage. Glucose, the main energy source, is broken down into adenosine triphosphate (ATP) and pyruvate in a process known as pyrolysis. In the presence of oxygen, the pyruvate produced at the end of glycolysis is transformed in the subsequent citric acid cycle and respiratory chain into more ATP, carbon dioxide and water. As early as the 1920s, the biochemist Otto Heinrich Warburg discovered that tumour cells ferment pyruvate to lactate (the anion of lactic acid), even in the presence of abundant oxygen (ed. note: Warburg effect, also known as aerobic glycolysis). Normally, this process only takes place under anaerobic (absence of oxygen) conditions, for example in muscles during extreme physical exertion. "Although this produces less ATP, the cancer cells can nevertheless produce the many building blocks they need for their rapid growth," explains Dr. Claus Kremoser, CEO of WMT AG. WMT stands for 'Warburg Metabolism Therapeutics' or simply 'We make cancer therapeutics'. The Heidelberg-based company develops new therapies that specifically interfere with the metabolism of cancer cells.
As a result of the Warburg effect, tumour cells consume large amounts of glucose and produce large amounts of lactate, which, along with protons (H+), is transported out of the cells via the monocarboxylate transporters MCT-1 and MCT-4. In most tumours, this process leads to the acidification of the tumours’ microenvironment, for example, in the low-oxygen (hypoxic) zones of solid tumours and in leukaemias and lymphomas often in the bone marrow. Along with high lactate levels, microenvironment acidification creates an immunosuppressive protective coat around the cancer cells that inhibits the activity of natural killer cells or tumour-specific T lymphocytes while attracting immunosuppressive immune cells such as myeloid suppressor cells, regulatory T cells or tumour-associated macrophages. Among other mechanisms, lactate therefore contributes significantly to the tumours’ ability to evade immune system attacks (camouflage). Furthermore, the Warburg metabolism and the high levels of lactate associated with it, reduces the efficacy of radio- and chemotherapy.
"Many cancer therapies are ineffective against solid tumours because the tumours slow down the existing immune response. This is why we want to readjust the ‘regulating screws’ of the tumours," says Kremoser, outlining the goal of the relatively new company, founded in 2020. The biochemist and his co-founders have many years of experience in the discovery and development of small drug molecules from their time at Phenex Pharmaceuticals AG. "We are now concentrating on the Warburg metabolism and want to interfere with it in such a way that immunoreactive cells will be able to recognise and destroy tumours."
Initial approaches that either directly inhibited the synthesis of lactate or associated signalling pathways led to severe side effects, as these processes also play a role in healthy cells. Switching off the transporters MCT-1 or MCT-4 reduced lactate excretion by only 50 percent, which does not have a high enough therapeutic efficacy. During their comprehensive investigations, the WMT researchers then came across an active ingredient, information about which was previously published by the Institute of Cancer Research (ICR) in London. This active ingredient, which is actually an inhibitor of the small nuclear protein pirin, is found in almost all cells.
However, more detailed analyses showed that the active ingredient also slowed down lactate production after the removal of pirin and that the inhibiting effect must be mediated accordingly via a different target molecule. Kremoser says: "The active substance can be easily dosed and has shown a clear anti-cancer effect in animal models. It massively slows down the Warburg metabolism without harming the animal. Unfortunately, our company did not own the active substance and therefore we couldn’t do much with it."
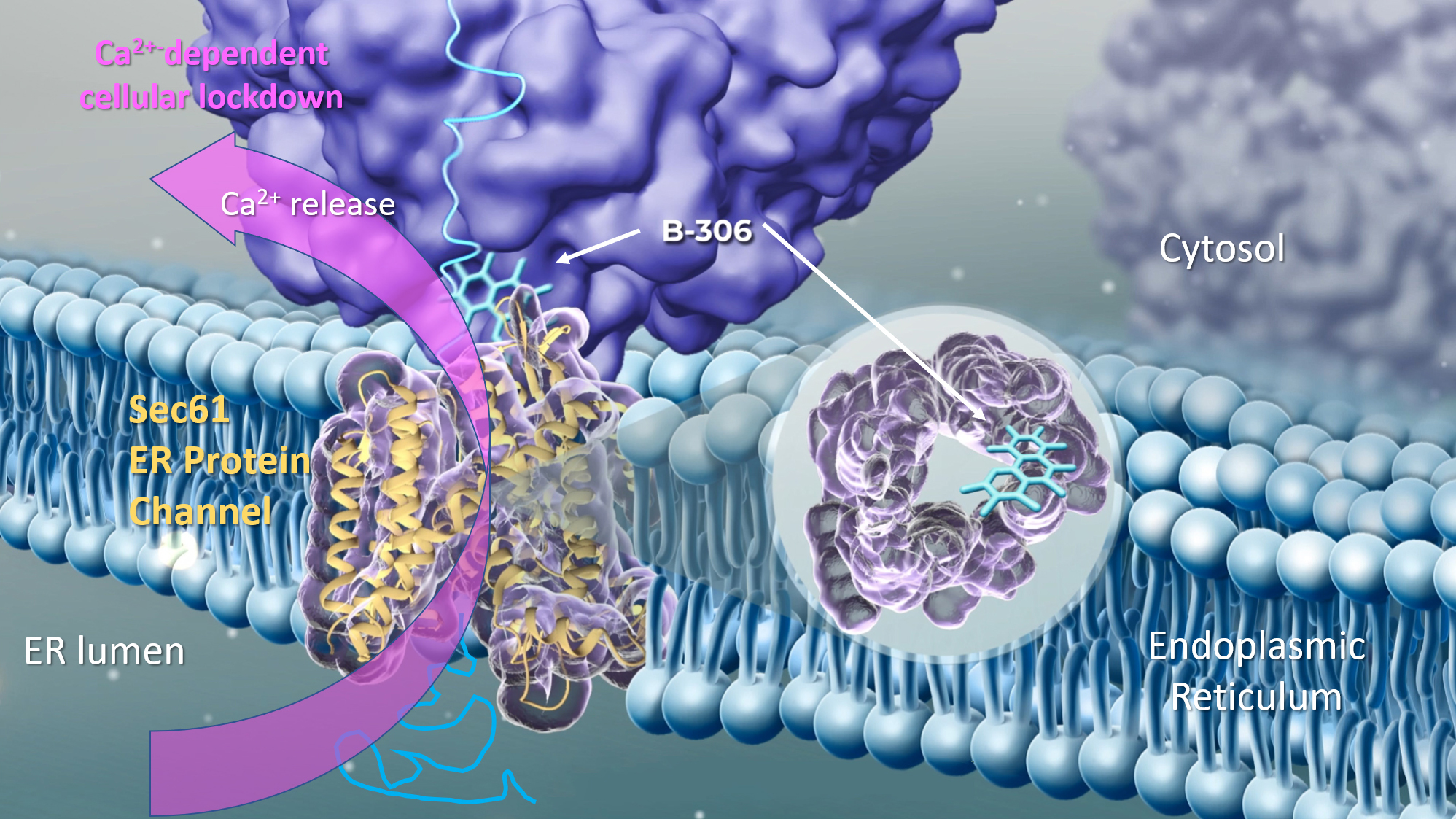 The modulator B-306 binds to the Sec61 tronslocon, a protein channel in the ER membrane, and induces its conformational change. This reduces the protein export rate, but also leads to the release of calcium ions (Ca2+) into the cytosol. This causes a general lockdown of cellular activities. The cancer cells cannot continue to grow and no longer secrete lactate, which changes the tumour microenvironment in such a way that the tumour is once again vulnerable to immune cells. © WMT AG
The modulator B-306 binds to the Sec61 tronslocon, a protein channel in the ER membrane, and induces its conformational change. This reduces the protein export rate, but also leads to the release of calcium ions (Ca2+) into the cytosol. This causes a general lockdown of cellular activities. The cancer cells cannot continue to grow and no longer secrete lactate, which changes the tumour microenvironment in such a way that the tumour is once again vulnerable to immune cells. © WMT AGIn the past two years, WMT has therefore carried out a lot of research and, through the clever selection of cellular assays, has been able to identify several substances that act either similarly to or better than ICR’s substance. However, in order to be able to attract new investors, the researchers also had to identify the target protein and decipher the mechanism of action. They achieved this in spring 2022 through a detailed analysis of the RNA signature, i.e. changes in the mRNA profile following the administration of the active substance. Biochemical interaction studies were carried out and it turned out that the substances targeted the Sec61 translocon, a protein complex found in the membrane of the endoplasmic reticulum (ER). Sec61 is a pore, which kind of resembles a revolving door, through which nascent proteins are translocated across the ER when they are ready for export. However, the substances identified by the company do not block the translocon completely, but just impair it, which leads to a significantly reduced protein export rate and, above all, to a strong leakage of calcium ions (Ca2+) from the ER into the cytosol. This induces a Ca2+-dependent signalling cascade inside the cell, which causes the ‘emergency shutdown’ of the cells and is associated with the impairment of the Warburg metabolism.
But wouldn't it make more sense to destroy the tumour cells directly? "In principle, yes, but if you want to be kind of selective, the way we have chosen is much better," Kremoser explains. "The translocon is a central and universal protein. But due to the fact that cancer cells have a much higher metabolic turnover and protein export rate, our substance has a particularly good effect on the cancer cells. When solid tumour cells are destroyed, they burst open and immediately release large amounts of lactate. This inhibits the immune system to an even greater extent. However, dormant tumour cells without a protective lactate coat can be recognised and destroyed." Chemical modifications can be used to influence the effect of the substances in such a way that the export of immunosuppressive signalling molecules and proteins such as PD-L1 and CD47 is reduced. "With a single small-molecule active substance that can be administered in the form of a tablet, two important, complementary effects can be achieved: on the one hand, tumour growth can be stopped, and on the other, the immune system can attack the tumour again. This is exactly what we have observed in the animal model."
WMT has further achieved an additional 20-fold increase in the selectivity of the active substances for cancer tissue by exploiting special physicochemical properties, thus enabling good dosing in vivo. The company was able to select the best molecule from over 330 active molecule variants synthesized over the past two years by comparing the molecules’ efficacy and toxicity. In October 2022, WMT was finally able to come up with a clinical development candidate – B-306 – with which clinical trials will be conducted once preliminary tests have been completed. B-306 will be used specifically for treating tumours with a high protein secretion rate, such as pancreatic and ovarian carcinomas or blood cancers like multiple myeloma and various B-cell lymphomas whose cancer cells originate from the bone marrow. Since the active substance is based on a universal anti-tumour principle rather than designed for a specific type of tumour, once the substance has been approved, there is hope for broader applications in the future.