Theranostics of prostate cancer: the combination of radionuclide diagnostics and radionuclide therapy
Using a low-level radiopharmaceutical that binds to the prostate-specific membrane antigen (PSMA), positron emission tomography/computed tomography can be used to visualise even small prostate cancer metastases. Developed by Heidelberg researchers, the radiopharmaceutical is a modified radionuclide diagnostic agent that has been coupled with a powerful emitter and used as a therapeutic tracer to irradiate and destroy cancer cells from within.
With more than 60,000 new cases annually, prostate cancer is the most common cancer among men in Germany, and with about 15,000 deaths (as of 2020), it is the second cause of cancer deaths after lung cancer. Mortality depends on the early detection and treatment of cancer before distant metastases are formed. For advanced metastatic prostate cancer, the likelihood of surviving five years after diagnosis is only about 30 percent.
A new radiopharmaceutical to improve detection of prostate cancer
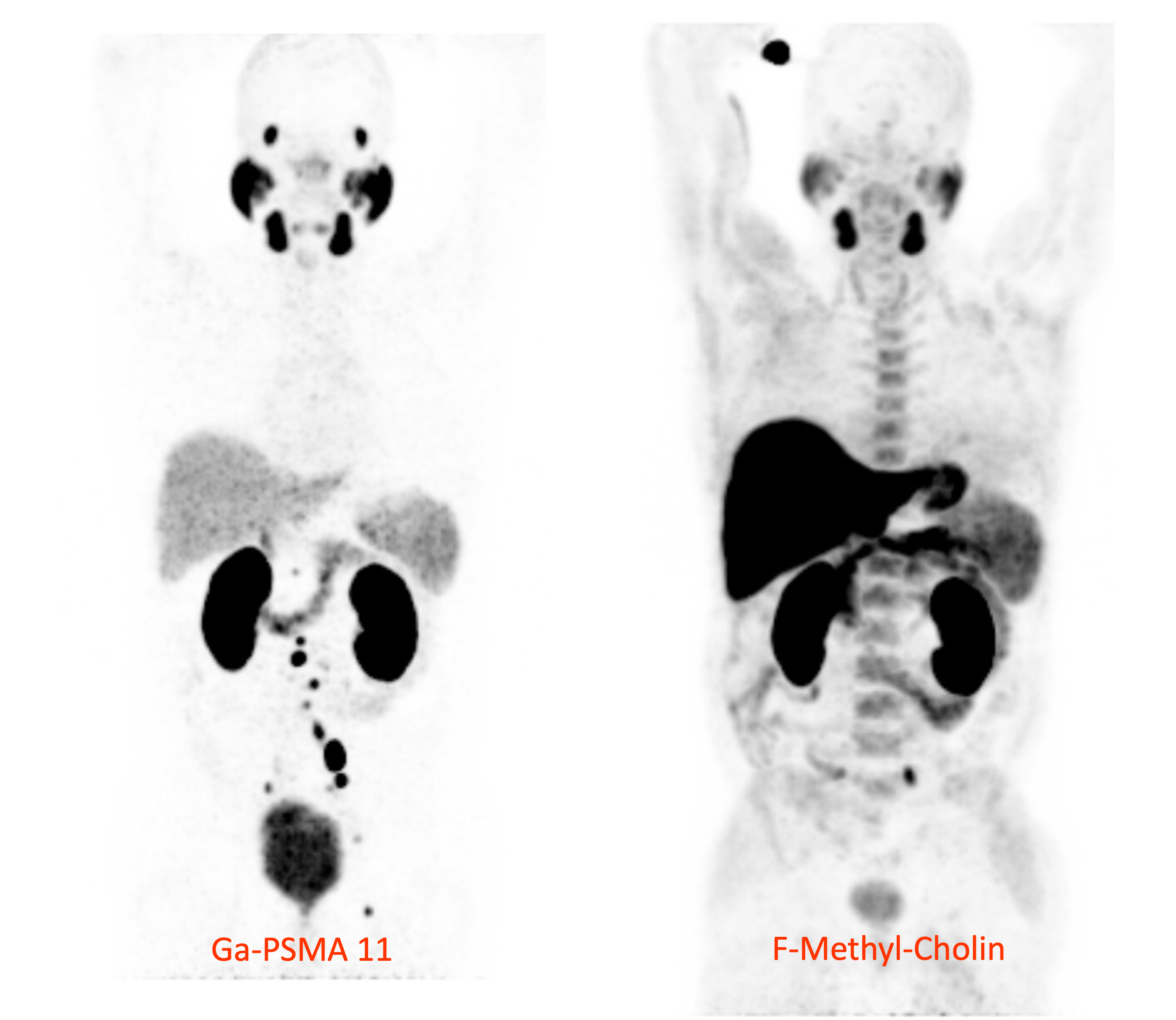 The 68Ga-PSMA-PET method can detect significantly more lymph node metastases of prostate cancer than the previously used 18F-choline PET method. © U. Haberkorn, Heidelberg University Hospital
The 68Ga-PSMA-PET method can detect significantly more lymph node metastases of prostate cancer than the previously used 18F-choline PET method. © U. Haberkorn, Heidelberg University HospitalIn 2009/10, a team led by chemist Prof. Dr. Michael Eisenhut, now director emeritus of the Division of Radiopharmaceutical Chemistry at the German Cancer Research Center (DKFZ) in Heidelberg, developed a molecule to diagnose prostate cancer and its metastases. The molecule in question binds to the prostate-specific membrane antigen (PSMA) and was later named PSMA-11. This synthetic ligand was coupled with the radionuclide gallium-68 (68Ga) to form a tracer molecule for diagnostic imaging using positron emission tomography/computed tomography (PET/CT). PSMA is a glycoprotein on the surface of prostate epithelial cells that is upregulated in all stages of prostate cancer. It occurs on cancer cells where it is amplified hundreds to thousands of times; PSMA is rarely found on other types of cells in the human body. The PET tracer [68Ga]Ga-PSMA-11 is injected into the bloodstream, bound by PSMA on the surface of cancer cells, and taken up into the cells where it accumulates. Non-invasive nuclear PET imaging is used to localise the gamma radiation produced by the positron decay of 68Ga in the cells.
 Prof. Dr. Uwe Haberkorn, Medical Director of the Nuclear Medicine Unit in the Department of Radiology at Heidelberg University Hospital, Head of the Clinical Cooperation Unit Nuclear Medicine at the German Cancer Research Center, Heidelberg. © U. Haberkorn
Prof. Dr. Uwe Haberkorn, Medical Director of the Nuclear Medicine Unit in the Department of Radiology at Heidelberg University Hospital, Head of the Clinical Cooperation Unit Nuclear Medicine at the German Cancer Research Center, Heidelberg. © U. HaberkornThe development of the radiopharmaceutical for use in radionuclide diagnostics was primarily the work of PhD student Martina Benesova (now head of the DKFZ Bayer Junior Research Group Molecular Biology of Systemic Radiotherapy), postdoc Dr. Matthias Eder (now professor at Freiburg University Medical Centre) and chemical engineer Martin Schäfer at the DKFZ. The preclinical animal experiments were performed by a team led by nuclear medicine expert Prof. Dr. Uwe Haberkorn in the Department of Radiology at Heidelberg University Hospital. Haberkorn introduced the PET tracer to the clinic back in May 2011 and tested it on individual patients for the first time.
As Eisenhut's successor, Prof. Dr. Klaus Kopka then took over, working with his team on the further development of [68Ga]Ga-PSMA-11 for use in molecular imaging. Kopka, who has been director of the Institute of Radiopharmaceutical Cancer Research in Dresden since 2019, describes the investigational results of a clinical trial with the PET tracer in patients at high risk of prostate cancer (funded by the German Consortium for Translational Cancer Research) as spectacular: "It makes visible even small metastases in the patient's body that would have been virtually undetectable with even the best computed tomography or magnetic resonance imaging method." The images of cancer metastases were of a much higher sensitivity than those produced using 18F-choline PET diagnostics, which was previously the gold standard. Haberkorn recalls. "The radiation therapists and the urologists immediately told me that they wanted to work exclusively with this new agent for diagnosis." The new method has since been used in hundreds of thousands of patients worldwide.
Development of a therapeutic tracer
 Prof. Dr. Klaus Kopka has been director of the Institute of Radiopharmaceutical Cancer Research, Helmholtz Centre Dresden-Rossendorf since 2019 (formerly at the DKFZ in Heidelberg) © K. Kopka
Prof. Dr. Klaus Kopka has been director of the Institute of Radiopharmaceutical Cancer Research, Helmholtz Centre Dresden-Rossendorf since 2019 (formerly at the DKFZ in Heidelberg) © K. KopkaAs previously mentioned, the tracer is selectively taken up by PSMA-bearing tumour cells and it was this property that prompted researchers at the DKFZ and Heidelberg University Hospital to transform the radionuclide diagnostic agent into a radionuclide therapeutic agent whose radioactive part not only ensures that the accumulations of the molecule in the cancer cells are visible, but also destroys them from within. The new variant of the PSMA-11 molecule was equipped with a radionuclide that emits hard, destructive short-range radiation. This resulted in the therapeutic tracer PSMA-617, which is coupled with the lutetium isotope 177Lu, a beta emitter.
Since the radiation from the radioisotope works over very short distances (a few millimetres) and the radiopharmaceutical is quickly removed from the bloodstream and excreted, the agent’s destructive effect is largely limited to the cancer cells to which it binds. Damage to surrounding cells is thus limited. Patients with numerous metastases throughout their body who no longer responded to other therapies were treated for compassionate use with the new compound [177Lu]Lu-PSMA-617, and the results exceeded expectations: whereas before treatment the patients had a life expectancy of a few months at best, in individual cases they were now able to survive for many more months or even years.
The theranostics concept
Chemists Eisenhut and Kopka, nuclear physician Haberkorn and biotechnologist Eder were jointly awarded the Erwin Schrödinger Prize in 2018 for their interdisciplinary development of radionuclide diagnostics combined with radionuclide therapy. The prize is awarded annually by the Stifterverband of the Helmholtz Association of German Research Centres for outstanding scientific achievements and technological innovations at the interface between the frontiers of medicine, engineering and the natural sciences.
The close interlocking of diagnostics and therapy, as seen in PSMA-11/PSMA-617 treatment, is referred to as theranostics (also: theragnostics), a concept that has great hopes riding on it, especially in the field of radionuclide therapy. In principle, specific molecules can also be developed against other tumour markers and these can be coupled to different emitters. Haberkorn and his colleagues have now completed their work on a class of tracers that recognises the protein FAP (fibroblast activation protein). FAP is found highly amplified in many advanced-stage human cancers on the surface of connective tissue cells associated with the tumours. It was called the ‘potential novel molecule of the century’ in a recently published review (Frontiers in Oncology, May 2022) and is already being tested in clinics at many different sites.
Approval of [177Lu]Lu-PSMA-617 for endoradiotherapy
The DKFZ had exclusively outlicensed to ABX GmbH in Radeberg for use in preclinical development. In 2017, the US company Endocyte Inc. acquired the licensing rights and began a Phase III clinical trial (VISION Trial) for approval as radioligand therapy against metastatic prostate cancer. In late 2018, Endocyte was acquired by Novartis for $2.1 billion. The Swiss pharma giant expects the drug to achieve annual sales of more than $1 billion. In late March 2022, the U.S. Food and Drug Administration (FDA) approved the drug under the name PluvictoTM for the treatment of metastatic (termed ‘castration-resistant’) prostate cancer in men who had received prior chemotherapy and who do not respond to hormone deprivation. When seeking approval with the FDA, the company had to prove that PSMA was present on cancer cells, which turned out to be the case in around 80 to 90 percent of cancer patients. When Prof. Dr. Michael Baumann, Chairman and Scientific Director of the DKFZ, heard the news, he said: "The FDA approval is a great opportunity for the affected men. Patients with advanced prostate cancer who otherwise have few promising treatment options can now benefit from the new drug worldwide." Approval by the European Medicines Agency (EMA) is expected shortly.