Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
A rapid pyrogen test: the human immune system as model
Every year, around 11 million people die of sepsis (blood poisoning) caused by microorganisms or microbial residues, known as pyrogens, entering the bloodstream. The smallest amounts can trigger fever. Researchers from the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart have developed a pyrogen test that does not require a laboratory and is not tested on animals. It is expected to be placed on the market soon.
 Dr. Anke Burger-Kentischer has been working on pyrogen test systems at the Fraunhofer IGB for more than 15 years. © BIOPRO Baden Württemberg GmbH, Photo: Braitmaier
Dr. Anke Burger-Kentischer has been working on pyrogen test systems at the Fraunhofer IGB for more than 15 years. © BIOPRO Baden Württemberg GmbH, Photo: BraitmaierBacteria, viruses or fungi are detected from components of bacterial cell walls, characteristic sugar and protein structures or the genetic material of the microbes by patrolling cells of the innate immune system. Receptors of immune and other body cells recognise the microbial molecular patterns. This interaction triggers the production of messenger substances that cause inflammation and fever. "This is our immune system’s first line of defence," explains Anke Burger-Kentischer, head of the Cell and Tissue Technology field of innovation at the Fraunhofer IGB in Stuttgart.
The pyrogen test that Burger-Kentischer’s research group has developed is based on an immune receptor subset that recognises the endotoxins of a large group of so-called gram-negative bacteria, i.e. lipopolysaccharides (LPS) in the bacterial cell wall. The test works in the same way as a pregnancy test: a few drops of sample solution are applied to the application window of the ImmuStick, and capillary flow transports them to the receptors on the strip.
If the sample contains bacterial endotoxins, the latter will bind to the corresponding immune receptor, thereby displacing a labelled molecule that has a much lower receptor-binding capacity than the pyrogens. The ligands are released and migrate with the labelled control molecules through the test strip where they become visible as coloured stripes in the viewing window. Two stripes appear in the viewing window if ligands and control molecules have been captured by the antibodies: one stripe indicates whether the sample contains pyrogens; the second stripe serves as confirmation that the test has worked, and should always appear.
Modular test principle
"The special thing about the pyrogen test is that we can use this immune receptor family in a modular way," says Burger-Kentischer. Depending on the receptors used, the test would also be able to detect gram-positive bacteria, viruses and fungi. Until now, pyrogens have only been detectable using more complex laboratory tests or animal experiments.
A conventional indirect pyrogen test uses human blood or the immune cells in blood and is carried out in a laboratory. A test substance is added and the number of fever-causing messenger substances released is measured after a short incubation time. Another method uses horseshoe crab blood, which clots when it comes into contact with an endotoxin.
"Animal testing is often not transferable to humans"
The oldest pyrogen detection system involves rabbits that are injected with the solution to be tested and are subsequently examined to see if they have developed a fever. "However, these animal experiments are often not transferable to humans because the animals’ immune system produces different results from the human immune system," says the molecular biologist. It therefore makes sense to use the receptors from the innate human immune system for the pyrogen test. "This not only reduces the number of animals used for pyrogen testing, but is also bio-intelligent," she emphasizes.
The researchers led by Burger-Kentischer developed and applied for a patent for a cell-based pyrogen test. The researchers genetically modified the cells in such a way that they presented human immune receptors as pyrogen detectors. However, this also required the tests to be carried out in laboratories. "My vision was to develop a simple positive-negative test that anyone can use anywhere."
Pyrogen testing is mandatory
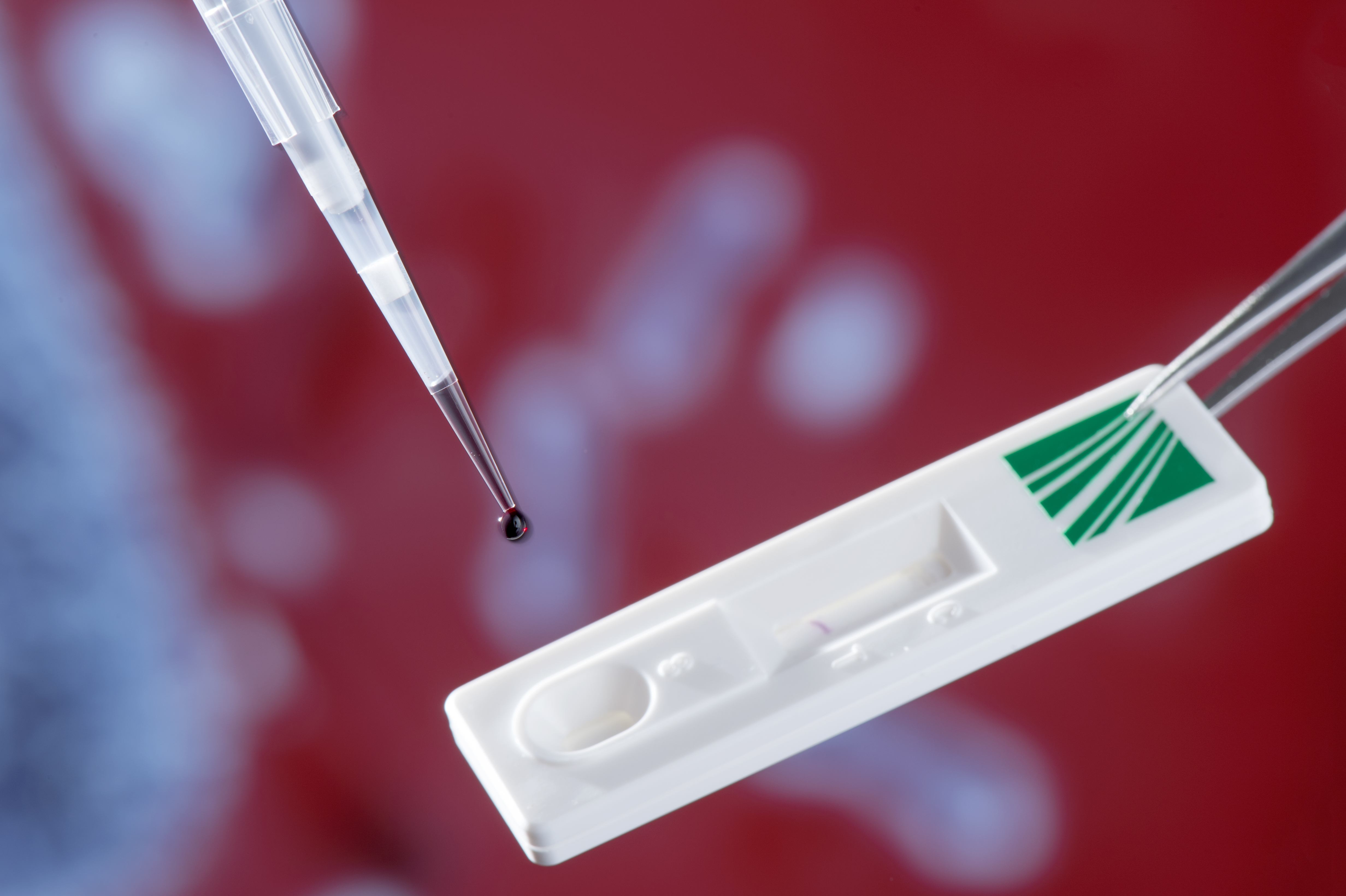 The first prototype of the pyrogen test measures approximately two centimetres by six centimetres. © Fraunhofer IGB
The first prototype of the pyrogen test measures approximately two centimetres by six centimetres. © Fraunhofer IGBThe ImmuStick can for example be used for checking the quality of drinking water in bathing lakes. Pharmaceutical companies and medical device manufacturers are even legally obliged to prove that their products are pyrogen-free to ensure that patients are not infected at a later date.
The Stuttgart researchers have since been granted a patent for the ImmuStick pyrogen test strip. "We were able to show that the test works well for bacteria and bacterial fragments in aqueous solutions," says Burger-Kentischer. Over the next three years, it will be a matter of finding the "best, simplest and most sensitive" test variant.
One particular aim is to test how many receptors are required for high test sensitivity or how to combine immune receptors that are specific for different pyrogens. Burger-Kentischer explained that her group was also looking into alternatives to the detection using the competitive displacement of ligands.
Preparing for market entry alongside industrial partners
In July 2019, the research group entered into a cooperation with three industrial partners that have experience in fluorescence labelling as well as the construction and marketing of test strips. “A product has to be completely mature before you can sell it. And this can only be done with cooperation partners,” says Burger-Kentischer.
In the long term, the biologist wants to use the test to prevent pyrogens from getting into the body. She also hopes to track down pyrogens that are already in the bloodstream. At present, it takes a long time to identify the microorganism that has caused an individual case of sepsis in order to be able to specifically treat the disease. This is why sepsis treatment usually starts with broad-spectrum antibiotics, which do not always work due to the growing number of bacteria that are becoming resistant to antibiotics. “The rapid test would enable doctors to prescribe antibiotics that treat the disease much more effectively if they could use the test to narrow the large number of bacteria down to a specific group, for example gram-negative bacteria,” says Burger-Kentischer. This might also help reduce the number of deaths from sepsis.