AI-supported image recognition accelerates identification of zebrafish mutants
EmbryoNet AI automatically identifies developmental disorders
In complex organisms, embryonic development is tightly regulated by intricate signalling pathways. When these pathways are disrupted, they can lead to characteristic developmental defects that are not always easy to detect with the naked eye. Developmental biologist Prof. Dr. Patrick Müller from the University of Konstanz has developed EmbryoNet, an AI-powered software tool that uses image analysis to reliably identify such developmental disorders.
During zebrafish embryogenesis - from a single egg to a complex organism - evolutionarily conserved signalling pathways orchestrate body formation in a highly coordinated, multilayered process. In the early stages of development, just a few key signalling pathways regulate tissue growth, cell differentiation and morphogenesis - the shaping of organs and body structures - in a manner not unlike a precisely choreographed dance. From what starts as an apparently disordered cluster of cells, distinct patterns emerge: the body axes, segmentation and individual body structures start to take shape. Signalling molecules, acting as conductors of this developmental ‘symphony’, assign immature cells their specific positions, guiding them to adopt their intended identities.
Cells ‘know’ where they belong
 Prof. Dr. Patrick Müller and his team at the University of Konstanz are developing an AI-powered tool designed to automatically analyse and characterise embryos and organoids. © Inka Reiter
Prof. Dr. Patrick Müller and his team at the University of Konstanz are developing an AI-powered tool designed to automatically analyse and characterise embryos and organoids. © Inka ReiterSignalling pathways interact within complex networks, influencing one another to guide differentiating cells into organised three-dimensional structures. A loss of function in any one of these pathways typically results in characteristic defects in pattern formation and consequently in the overall shape of the developing embryo. Developmental biologist Prof. Dr. Patrick Müller from the University of Konstanz investigates these morphological changes in vertebrate models such as zebrafish. By studying specific mutants, his team can identify which signalling pathways might be disrupted and how they contribute to developmental abnormalities. "If you alter gene regulation by manipulating signalling molecules, the embryo undergoes characteristic shape changes - some striking, others more subtle," says Prof. Dr. Patrick Müller. This variability makes characterisation using image analysis challenging, and drawing clear conclusions can often be difficult. His solution: to standardise embryo characterisation using artificial intelligence, which enables high-throughput analysis. "We focused on a set of evolutionarily conserved signalling molecules that are activated during early development and reused in adult organisms as well as in disease states," Müller explains. With a powerful AI-based tool capable of analysing embryonic shape and structure, it becomes significantly easier to investigate how certain substances act at the molecular level, thus rendering the risk assessment of their impact on biological development more efficient.
AI as a key component
 Artificial intelligence and biology: a visualisation of the scientific research driving the development of the EmbryoNet AI platform. © Anton Startsev with MidJourney
Artificial intelligence and biology: a visualisation of the scientific research driving the development of the EmbryoNet AI platform. © Anton Startsev with MidJourneyThe EmbryoNet software developed by Prof. Dr. Patrick Müller and his team combines image data with temporal information on embryonic development, enabling developmental disorders in embryos to be reliably classified. The precision of the analysis is significantly increased by incorporating time series data. "We can also capture developmental dynamics," explains Müller. "For instance, whether the embryo is developing at the normal pace or if it is delayed."
More than 15 million images - amounting to several terabytes of data - have been fed into the artificial intelligence system developed by Müller and his team. At the heart of EmbryoNet is a convolutional neural network (CNN), a type of deep learning architecture that consists of multiple layers, each with distinct filters and focal points that interact to process complex image data. "At the end of the network, the system performs classification. These networks are inspired by biological brain processes and closely mimic how the brain interprets visual input," explains Müller. The method was first published in Nature Methods in 2023 and featured as part of the journal’s Method of the Year1).
Unbeatable accuracy and speed
Compared to human image assessment, EmbryoNet is significantly less biased and far more robust in identifying zebrafish mutants. It also operates significantly faster: the AI can analyse several hundred images in just milliseconds whereas processing the same dataset would take human experts several days. In terms of accuracy, EmbryoNet achieves a remarkable 90% classification precision - surpassing experienced developmental biologists, who typically achieve 80%, and far exceeding the 50% accuracy of trained students.The system is both highly precise and versatile: its application extends beyond zebrafish to other vertebrate species and organoids, making it a valuable tool for advancing animal-free research. Overall, EmbryoNet substantially increases research efficiency by detecting phenotypic abnormalities hours before they become visible to the human eye and even identifying instances of incomplete penetrance. "EmbryoNet clearly detects regions we were previously blind to. It opens the door to a new layer of biology that was simply inaccessible to us before," says Müller.
Customised solution for pharma and research
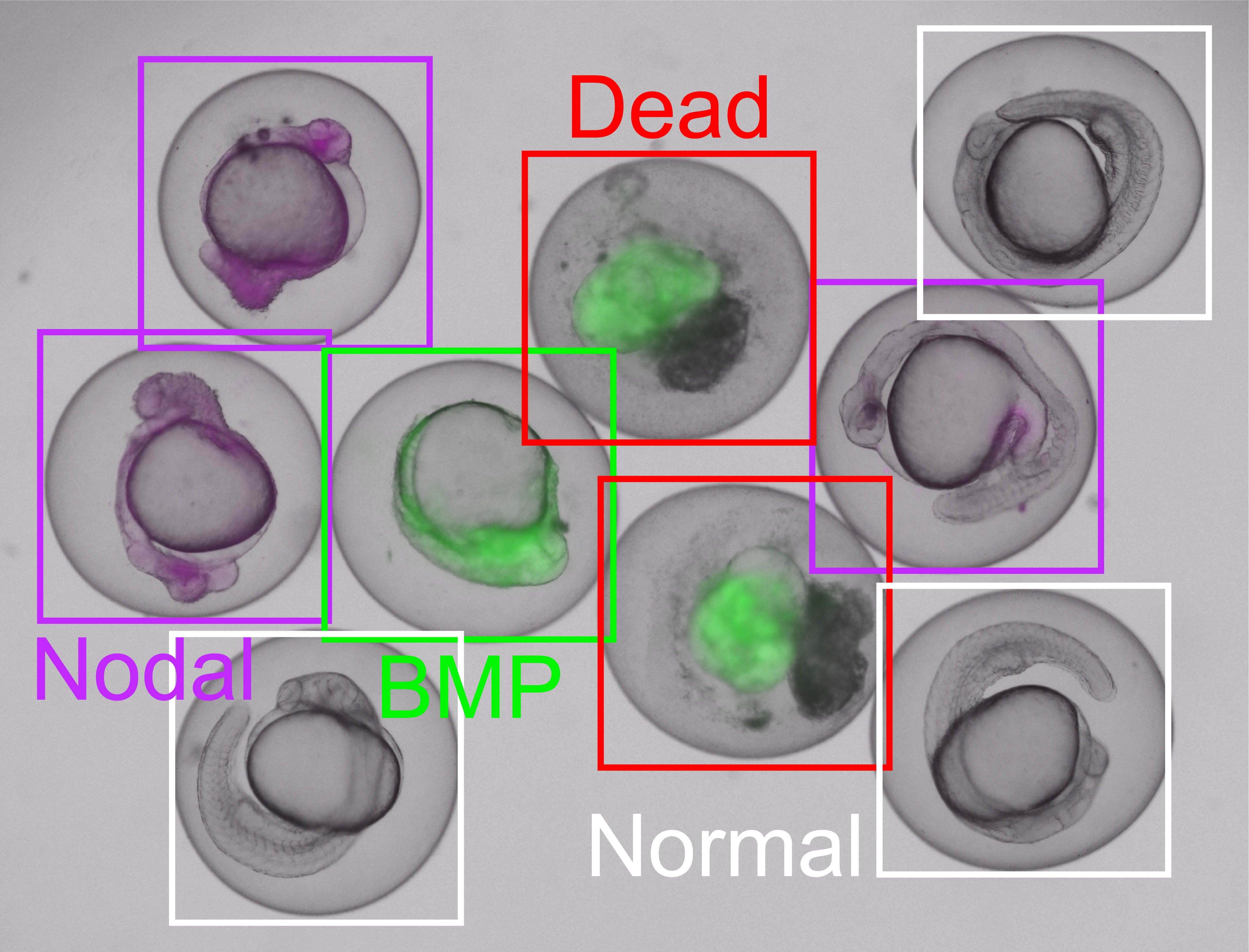 Developmental disorders - such as those observed in zebrafish - can be reliably identified and linked to the underlying signalling pathways, such as Nodal or BMP. EmbryoNet also distinguishes between normally developed embryos and non-viable (dead) ones with a high level of accuracy. © EmbryoNet AI
Developmental disorders - such as those observed in zebrafish - can be reliably identified and linked to the underlying signalling pathways, such as Nodal or BMP. EmbryoNet also distinguishes between normally developed embryos and non-viable (dead) ones with a high level of accuracy. © EmbryoNet AIUsing EmbryoNet, known drugs can be systematically linked to previously unrecognised effects on signalling pathways. In a recent drug screen, Prof. Dr. Patrick Müller's team discovered that statins - commonly used to lower cholesterol - also impact embryonic development. "The embryos exhibited a specific deformation with a shortened body axis, which is characteristic of disruptions in the FGF signalling pathway," explains Müller. "So we can detect that something is going wrong in embryogenesis, but also pinpoint what exactly is affected - namely, FGF signal transduction. This pathway is not only critical for early embryonic development but is also frequently dysregulated in cancer.” Müller is hoping that: "In future, targeted antagonists might be used to counteract such dysregulation - potentially even correcting developmental defects."
With his start-up, EmbryoNet AI Technologies, Prof. Dr. Patrick Müller aims to bring these innovations to the pharmaceutical industry. The platform is designed for ease of use as a web-based application and requires no special expertise. "You simply drag and drop an image taken in the lab into your browser, and the analysis is generated instantly. You’ll see the phenotype identified, any morphological abnormalities, tissue composition, and much more," Müller explains. To allow users to test the tool, up to ten images can be analysed free of charge. After that, annual licences are available - optionally bundled with a high-throughput microscope for larger-scale applications. EmbryoNet AI Technologies was awarded the Start-up Award 2025 by the German Federal Ministry for Economic Affairs and Climate Action (BMWK) for the successful research transfer of this technology, as well as a Proof of Concept Grant from the European Research Council (ERC).
While Müller and his team work towards gaining regulatory approval from the European Medicines Agency (EMA), he envisions EmbryoNet AI making a standardised contribution to biomedical research - one that boosts both reproducibility and relevance to human biology.