Cell-based solution in place of animal testing
Innovative cell-based test to detect pyrogens in drugs
Drug safety testing for microbial contaminants and fever-inducing substances (pyrogens) still frequently uses animal experiments. InnoZell, a start-up that will soon be spun off from the University of Konstanz, has developed a compelling alternative: an animal-free, human cell-based assay that is faster, more sensitive and more reliable than traditional methods – and can be customised to meet user-specific needs.
Even minimal impurities in pharmaceuticals can pose a serious risk to patient health. They must therefore be demonstrably free from by-products, degradation compounds and other unwanted substances. Many medicinal devices or instruments also undergo sterilisation – a process in which microorganisms are either eliminated through heat, gas or radiation treatment or removed via sterile filtration.
 (from left to right) The InnoZell team Erik Sontowski, Dr. Ann-Kathrin Mix and Dr. Clovis Hugues Seumen Tiogang developed the human cell-based test system CellAlarm for pyrogen detection.
(from left to right) The InnoZell team Erik Sontowski, Dr. Ann-Kathrin Mix and Dr. Clovis Hugues Seumen Tiogang developed the human cell-based test system CellAlarm for pyrogen detection.Even stricter safety regulations apply to parenteral drugs – medicines such as vaccines or infusion solutions that bypass the digestive system and need to be free from both viable microorganisms and fever-inducing substances known as pyrogens. The latter are often heat-stable components of the outer membrane of gram-negative bacteria known as endotoxins and can be released both by living bacteria or when bacteria are destroyed during the sterilisation process. However, endotoxins are not the only pyrogens. Other microbial components – from bacteria, viruses or fungi – as well as non-biological particles such as tiny plastic contaminants, can also trigger severe and potentially life-threatening fever reactions, even when present in minute amounts.
"Rabbits are still widely used for pyrogen testing," explains Dr. Clovis Hugues Seumen Tiogang of InnoZell, a University of Konstanz spin-off. "Every year, approximately 400,000 rabbits die worldwide as a result of these tests. We've now developed a human cell-based alternative that detects pyrogens faster and with greater sensitivity."
Animal-based pyrogen testing is standard
The rabbit pyrogen test (RPT) was introduced in the 1940s and remains one of the standard animal-based methods used in regulatory drug safety testing.¹) A pharmaceutical preparation is injected into rabbits, and the product is only deemed safe for use if no rise in body temperature occurs. While the RPT can detect a broad spectrum of pyrogens, its relevance to humans is limited, as rabbits do not always exhibit fever responses as sensitively or reliably as humans do.
The Limulus amoebocyte lysate (LAL) test, developed in the 1960s, is another animal-derived method. It is an in vitro test system that uses blood cells (amoebocytes) extracted from the horseshoe crab. An estimated 550,000 horseshoe crabs are used every year, ten to thirty percent of which fail to survive the blood collection process.²) A key limitation of the LAL test is that it only detects endotoxins, a specific class of pyrogens. The same applies to its modern alternative, the recombinant factor C (rFC) test, approved since 2021 and significantly more sensitive than the traditional rabbit pyrogen test (RPT).
Another alternative to the rabbit pyrogen test (RPT) is the monocyte activation test (MAT), which was incorporated into the European Pharmacopoeia in 2010. This in vitro assay simulates the human immune response to pyrogens by using human whole blood to detect both endotoxins and non-endotoxin pyrogens. When exposed to a fever-inducing substance, monocytes – a specific type of immune cell – are activated and release pro-inflammatory cytokines. A subsequent analysis then quantifies these signalling molecules and provides a sensitive and physiologically relevant measure of pyrogenic activity.
CellAlarm from InnoZell is faster and more sensitive
During his doctoral research, biochemist Seumen Tiogang focused on the negative regulatory mechanisms of the innate immune response. To better study how immune cells react on contact with microorganisms, he engineered several signalling pathways in a widely used monocyte cell line – commonly employed in MATs – and introduced a novel blue fluorescent reporter protein. 3) As he explains, "These modifications allow us to observe cellular responses to pyrogens within three rather than sixteen hours. The intensity of the reaction is directly measurable through the blue fluorescence, eliminating the need for additional analysis steps. It is a significantly more sensitive system than the original cell line."
In 2022, the young scientist’s doctoral supervisor, Prof. Dr. Christof Hauck, recognised the project’s significant potential for advancing animal-free drug testing and encouraged him to apply for the Universities of Konstanz start-up initiative, Kilometer1. Tiogang teamed up with his colleague Erik Sontowski, and together they won the Kilometer1 Award in the Science Innovation category with their product, CellAlarm.
In 2023, biologist Dr. Ann-Kathrin Mix joined the team, which is now preparing to launch a spin-off having secured funding through the University of Konstanz’s Excellence Strategy to advance the development of their product. In summer 2024, the trio was also awarded the special research prize from Unternehmer:innen für Gründer:innen (UfG e.V.), a non-profit organisation that fosters entrepreneurial innovation in the Konstanz region. Beyond financial support, the researchers have greatly benefitted from tailored business mentoring provided through these competitions and from collaborative exchanges within the Konstanz start-up network, Kilometer1.
Individual solutions for users
The exploratory phase, funded by the German Federal Ministry of Education and Research (BMBF) ‘GO-Bio initial’ programme until autumn 2025, focuses on the technological transfer to industry. "Over the past two years, we have made further modifications to the cell line so it can be used in a variety of ways," explains Mix. "We now have a platform that can respond to different user-specific needs." A patent for the innovative development has already been applied for.
The decision on which services to prioritise in the future was guided by a comprehensive market analysis. Sontowski explains, "Our core expertise currently lies in research and development. In the short term, we are focusing on product development and technology transfer for in-house customer use. Through close collaboration with users, we will continuously tailor our high-performance CellAlarm platform to meet their evolving needs."
Rabbit tests only permitted until the end of 2026
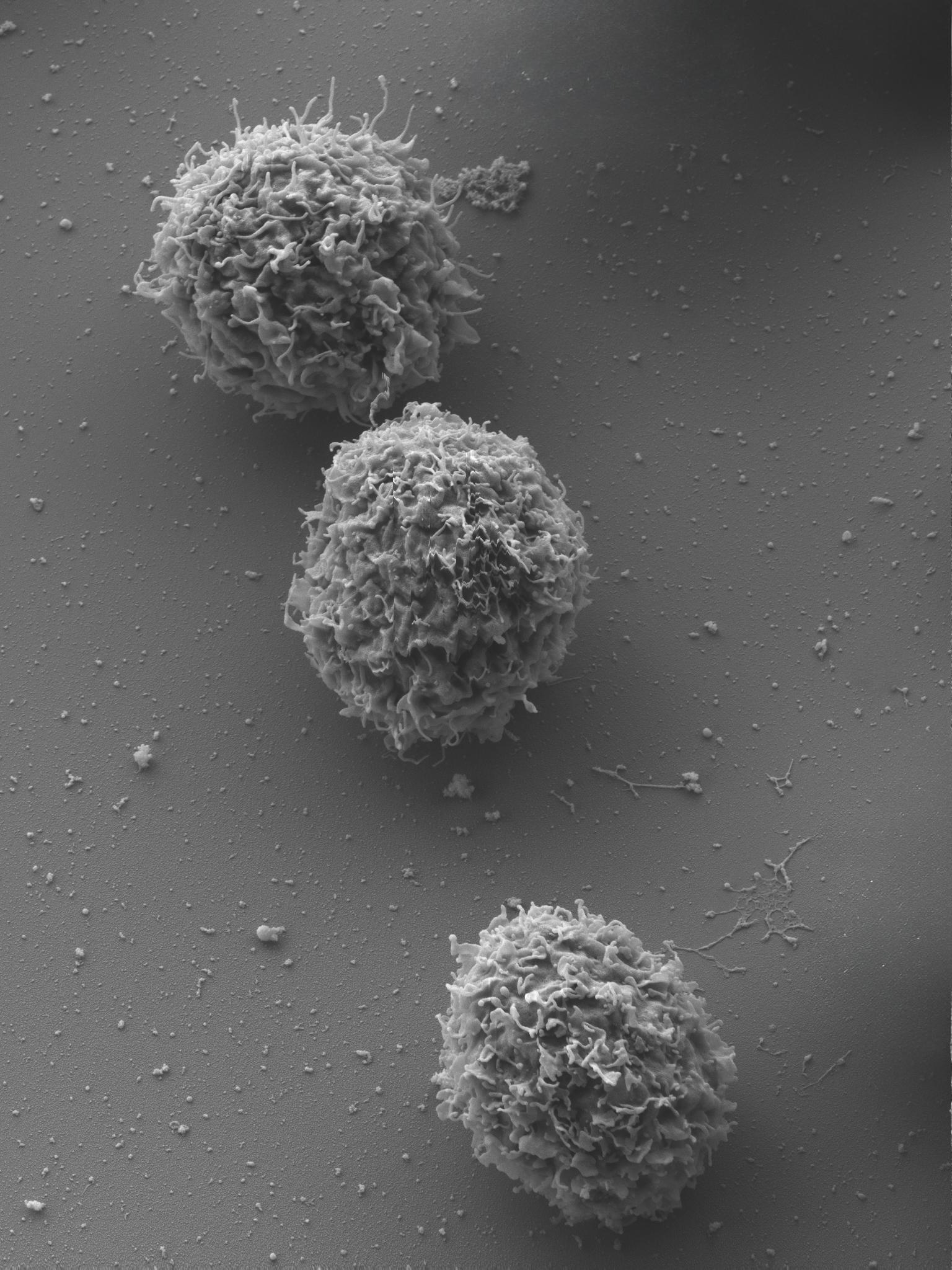 Scanning electron microscope image of the high-performance CellAlarm cells. © Michael Laumann/EMC/University of Konstanz
Scanning electron microscope image of the high-performance CellAlarm cells. © Michael Laumann/EMC/University of KonstanzThere is an urgent need for new animal-free testing methods. In accordance with the EU Directive 2010/63/EU, the rabbit pyrogen test (RPT) will be phased out from the European Pharmacopoeia starting in 2026 and will no longer be permitted for medical quality control. 4) Meanwhile, the demand for parenterally administered biopharmaceuticals – such as therapeutic antibodies, enzymes, hormones and vaccines – is on the increase.
As Mix explains, "Compared to the rabbit test and existing alternatives, our all-in-one product is more sensitive, faster, more reliable and also more cost-effective. Maintaining animals is very resource-intensive, and two-stage MATs demand additional time and personnel." Tiogang adds, "Most importantly, CellAlarm is more relevant to human biology, as rabbits respond inadequately to certain pyrogens that pose significant risk to humans."
The InnoZell team is currently seeking industrial partners to help realise the proof of concept. Given the test system’s versatility, each application requires separate validation. Accordingly, CellAlarm will be evaluated against conventional pyrogen detection methods using real samples across multiple projects. Follow-up funding for this phase has already been applied for in collaboration with the University of Konstanz. "Ongoing support from the university is crucial for us," says Sontowski. "We benefit from valuable scientific input and access to their laboratory facilities." Once he completes his doctoral thesis at the end of the year, all three team members will be able to dedicate themselves full-time to advancing the project. With sufficient relevant validation data, InnoZell will be launched to bring this innovative testing method to market in the near future.