Rewriting the transcript to heal diseases
Therapy involving the umlauts of the genetic alphabet
tRNAs are essential components of the protein synthesis machinery that also act as molecular switches in gene regulation and consequently in disease processes such as cancer. The Heidelberg-based start-up Umlaut.bio is developing novel therapeutics that specifically target tRNAs to intervene at the molecular origin of disease.
For a long time, the genetic code - composed of four bases, namely adenine (A), cytosine (C), guanine (G) and thymine (T) - was considered the blueprint of life and the sole source of information for cellular function. Since the development of increasingly powerful and detailed methods for investigating nucleic acids, it has become clear that gene expression is far more complex than originally assumed.
 Dr. Bastian Linder is CSO of the start-up Umlaut.bio and a long-standing expert in RNA research. © Umlaut.bio
Dr. Bastian Linder is CSO of the start-up Umlaut.bio and a long-standing expert in RNA research. © Umlaut.bioAlthough the DNA sequence is the foundation of cellular development processes, they are also significantly regulated by a second level of control - the epigenome - through modifications such as methylation, for example. However, something else is also involved: around a decade ago, a third level of regulation was discovered - the epitranscriptome. At this level, gene activity is influenced by chemical modifications of ribonucleic acids that are comparable to umlauts in a text, which do not fundamentally change the letters themselves but instead add a layer of nuance and fine-tuning.
The most common chemical RNA modification is m6A (N6-methyladenosine). It has long been suspected to regulate the stability and availability of mRNAs modified in this way, but beyond that, little was known. Dr. Bastian Linder, first as a postdoctoral researcher in Samie Jaffrey’s laboratory in New York and later at the European Molecular Biology Laboratory (EMBL) in Heidelberg, set out to investigate this by generating a comprehensive map of hundreds of mRNA nucleotides that carry this modification. "But even after we had mapped the nucleotides, their function remained unclear even though we had expected it to be more or less self-evident," he says.
messenger RNA (mRNA) vs. transfer RNA (tRNA)
Various ribonucleic acids are used for different tasks in the cell, including:
mRNA: Carries genetic information from DNA to the ribosomes and serves as a template for protein synthesis. It is a complementary copy of the gene sequence needed to produce a specific protein.
tRNA: Transports the appropriate amino acids - the building blocks of proteins - to the ribosomes. Each tRNA recognises the complementary codon on the mRNA via its anticodon, ensuring that amino acids are added in the correct order as specified by the genetic code.
Previously undiscovered level of gene regulation
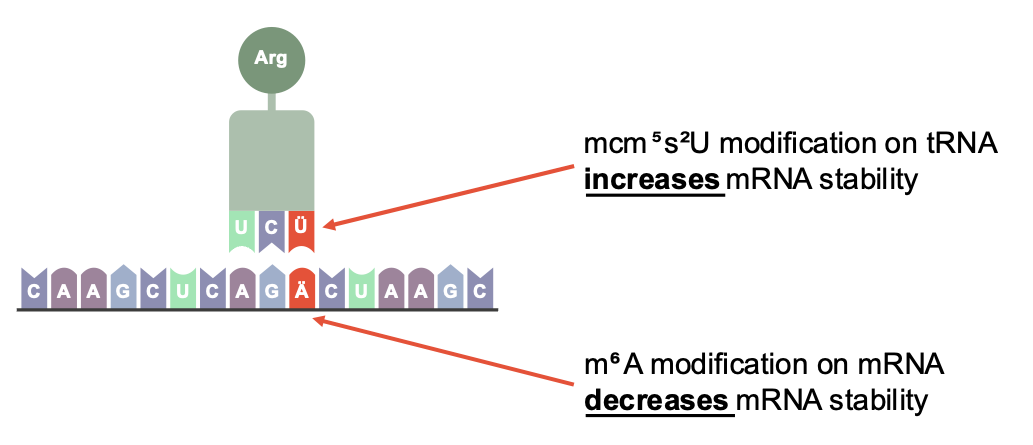 Schematic representation of post-transcriptional gene regulation: Modifications to both mRNA and tRNA can determine how stable transcripts are in the cell. © Umlaut.bio
Schematic representation of post-transcriptional gene regulation: Modifications to both mRNA and tRNA can determine how stable transcripts are in the cell. © Umlaut.bioAs mentioned above, until recently, the RNA modification m6A was only suspected of regulating the stability and availability of mRNAs. However, Linder and an international research team have now been able to demonstrate that this is indeed the case. m6A, when incorporated into the coding sequence of an mRNA, leads to accelerated mRNA degradation. This occurs because the modified codons are processed more slowly by ribosomes, causing translation to stall and ribosome collisions to occur - events that apparently signal mRNA degradation. However, at the same time, a specific tRNA [editor’s note: tRNA, or transfer RNA, transports the correct amino acid to the ribosome during translation, matching codons on the mRNA] modification, known as mcm⁵s²U (5-methoxycarbonylmethyl-2-thiouridine), can counteract this effect. This represents a completely new level of post-transcriptional gene regulation, as it shows that both mRNA modifications and tRNA modifications play a critical role in transcript stability. Together with the mRNA itself, these modifications influence how long an mRNA persists in the cell.1)
"This mechanism is used to regulate gene activity differently: housekeeping genes must have more stable mRNAs than the genes of signal transduction pathways," explains Linder. "In fact, this mechanism is often altered in diseases. For example, cancer cells exploit the overactivation of such pathways and stabilise the mRNAs of signalling molecule genes through modified tRNAs, enabling stronger and longer-lasting expression. In this way, they promote cell growth and activity. mcm⁵s²U and m6A therefore jointly control the expression of these derailed signalling pathways."
Restoring degenerated cells to a normal state
The realisation that certain RNA modifications - essentially the 'umlauts' of the genetic alphabet - can play a key role in pathological conditions gave the experts the idea to intervene in this system in order to steer signalling pathways back onto a healthy course. Together with biochemist and investment banker Dr. Karsten Fischer, Linder founded a biotechnology start-up based at BioLabs Heidelberg in 2024. They chose a memorable name for the EMBL spin-off: Umlaut.bio. Shortly afterward, the management team, consisting of Chief Executive Officer (CEO) Fischer and Chief Scientific Officer (CSO) Linder, was joined by medicinal chemist Dr. Simon Breitler as Chief Development Officer (CDO).
Umlaut.bio uses Linder's findings from RNA research to develop novel therapeutics that specifically target and block chemical modifications of tRNA, thereby inhibiting its ability to stabilise mRNA. This approach is intended to halt uncontrolled growth of cancer cells or the excessive activation seen in inflammatory and autoimmune diseases. "By controlling gene expression, we cause the cells to abandon their previous programme. The biology behind this is new and incredibly exciting. No one had ever considered this class of drugs before," says Linder.
Approach combines precision with broad spectrum of action
As in classic drug development, the experts began with small molecules that target the activity of RNA-modifying enzymes. Small molecules are chemical compounds with a relatively low molecular mass that can be used as drugs such as aspirin or ibuprofen. According to Linder, this approach was deliberately chosen to minimise risk. The current aim is to prove the concept in oncology. If successful, this could represent a fundamentally new and gentle therapeutic approach. The cancers initially being considered are not limited to a single type, because targeting tRNA modifications is intended to address disrupted gene regulation more broadly. Importantly, normal translation remains unaffected, allowing tRNA to continue fulfilling its essential functions. "There are already indications that this strategy can inhibit cancer cells," says the company founder.
The start-up completed high-throughput screening of small molecules a few weeks ago. "Together with a partner, we developed the system from scratch and established the screening process. This was an extremely difficult task," explains Linder. Although there is still a long way to go before these drugs can be used in humans, the goal of systemic application - ideally oral - is already in sight. "Our drugs will not be comparable to conventional chemotherapeutics, which are versatile but often come with many side effects," explains the molecular biologist. "They are more similar to precision medicine approaches that target tumours with very specific dependencies, which are also based on highly specific signalling pathways. We combine this knowledge and use our therapy to 'hit the brakes,' restoring the normal, natural signalling pathways. This approach is promising for many types of cancer, and we will be spoiled for choice when deciding where to start."
Once this is underway, the Umlaut team would like to pursue a second programme: developing approaches that target other tRNA modifications and additional indications, for example in immunology. However, first on the agenda is a new round of investment and fundraising. "We are doing everything we can to make the therapeutics available as quickly as possible," says Linder.