Advanced therapy medicinal products: gene and cell therapies
Novel gene and cell therapies for treating incurable and hereditary diseases have raised high expectations. However, success has so far been limited to the long-established bone marrow transplants involving the administration of haematopoietic stem cells used to treat blood cancer. CAR T-cell therapies have recently emerged as a major new hope in cancer treatment.
Gene therapy involves preventing or treating a disease caused by a defective gene. This is done by transferring appropriate nucleic acids into the affected cells to repair the relevant gene or by replacing it with a functional gene. Since interventions in the genes of germ cells or early embryonic cells, so-called germline gene therapies, are prohibited in Germany for ethical reasons, only ‘somatic gene therapy’, in which the therapeutic nucleic acid is introduced into somatic body cells, is discussed below. In somatic gene therapy, gene modification is restricted to the recipient and not passed on to any children.
Cell therapy involves taking defined cells that have previously been isolated and propagated in cell culture and transplanting them into the patient's diseased target organ. These cells will then take over and restore the organ’s functions. Depending on where the transplanted cells originate, a distinction is made between:
(i) Autologous transplants, in which the cells originate from the patients themselves, so that there is no need to fear rejection by the patients’ immune system. However, the quantities of available cells are often too small for clinical use.
(ii) To improve this situation, allogeneic transplants in which a person’s stem cells are replaced with healthy stem cells donated by someone else are usually performed. If possible, immunologically similar cell donors, e.g. close blood relatives, are used to reduce the risk of the patient's immune system rejecting the graft. Immunosuppressive treatment is nevertheless usually required. Even the transfer of cell lines from cell cultures is associated with a risk of rejection, which has to be countered by suppressing the patient’s immune system.
(iii) This is of course also true for xenogeneic transplantation, i.e. the transplantation of animal cells to humans.
As regards the transplantation of larger cell assemblies, as needed for example in skin transplantation in the case of burns, a distinction is also made between autologous, allogeneic or xenogeneic tissue therapies.
Stem cell therapies
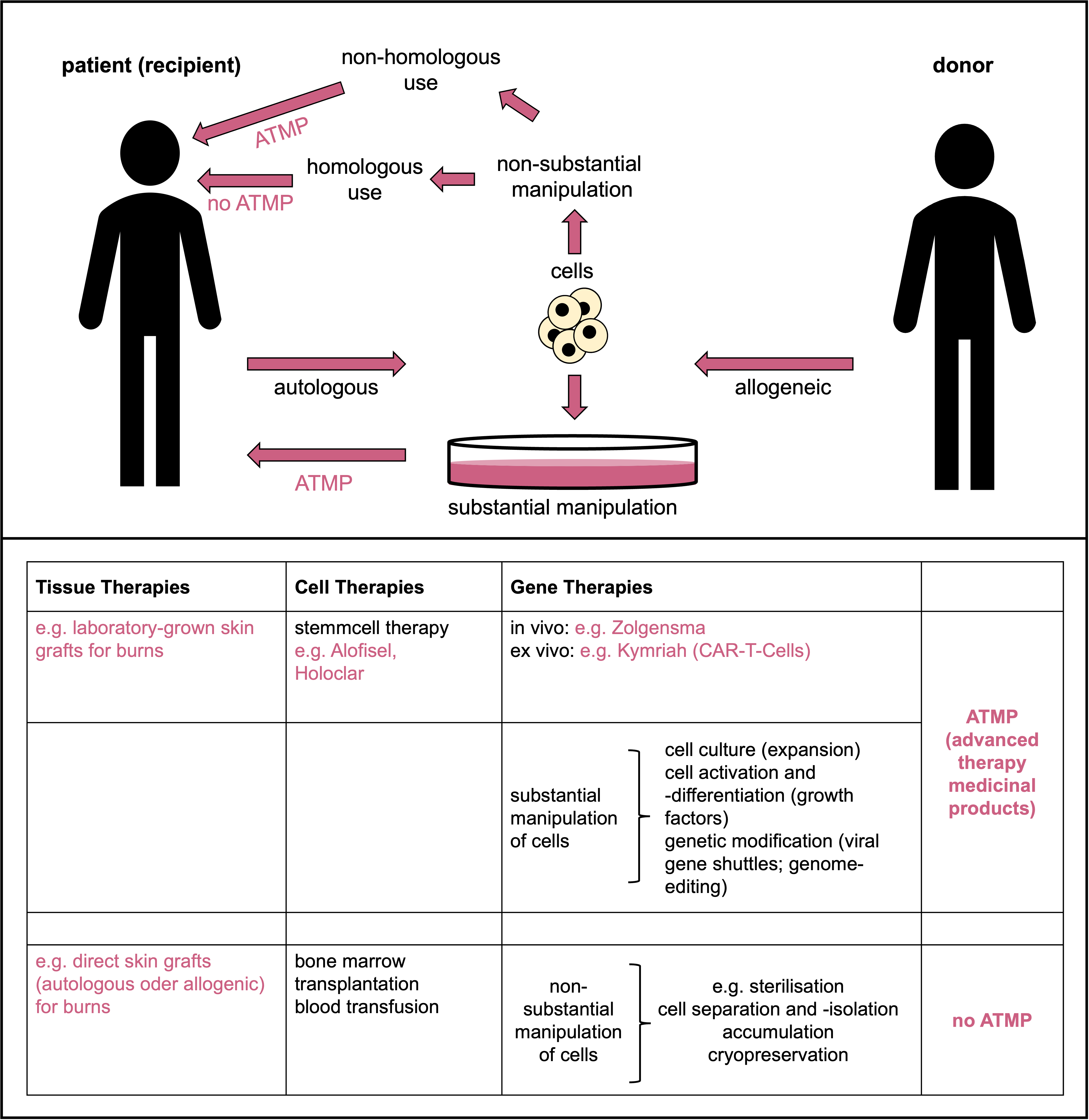 Schematic depicting the terms and their definitions used in tissue, cell and gene therapies and ATMPs. © Dr. Ernst-Dieter Jarasch (adapted from: Karin Hogedoorn & Heather Main 2020; https://atmpsweden.se; graphic implementation: Jana Friedl/BIOPRO Baden-Württemberg)
Schematic depicting the terms and their definitions used in tissue, cell and gene therapies and ATMPs. © Dr. Ernst-Dieter Jarasch (adapted from: Karin Hogedoorn & Heather Main 2020; https://atmpsweden.se; graphic implementation: Jana Friedl/BIOPRO Baden-Württemberg)Stem cells are important for the development and repair of organs and tissues; they therefore have great potential, for example, for treating degenerative diseases, which are becoming increasingly common in our ageing societies. This is particularly true for human embryonic stem (hES) cells, which can differentiate into all different cell types (over 200) in our body. However, deriving hES cell lines from fertilised human oocytes is prohibited in Germany by the Embryo Protection Law; the import of hES cells produced abroad is only permitted in exceptional cases and only for research purposes.
There were great hopes for induced pluripotent stem cells (iPS cells), which are artificially produced by reprogramming already differentiated somatic cells. The Japanese scientist Shinya Yamanaka was awarded the Nobel Prize in Physiology or Medicine for the development of iPS cells in 2012, and clinical trials with iPS cells are still being conducted, primarily in Japan. The first success was achieved with a nearly blind Japanese woman who had reprogrammed stem cells from her own skin transplanted into her eye.
Despite hundreds of clinical trials, there has been very limited progress with adult (somatic or tissue-specific) stem cells; efforts are mainly focused on treating heart attacks, Parkinson's disease, paraplegia and diabetes. The first stem cell therapy approved by the European Medicines Agency EMA (Holoclar for the regeneration of the cornea of severely damaged eyes) was launched in 2015. In March 2018, the EMA approved another somatic cell therapy (Alofisel) for treating Crohn's disease, a serious inflammatory bowel disease. The active ingredient of Alofisel is stem cells that have been isolated from the adipose tissue of donor individuals and propagated in vitro.
These rare successes stand in stark contrast to established therapies using haematopoietic (blood-forming) stem cells. These cells are derived from bone marrow and have long been used at many hospitals to treat certain leukaemias and lymphomas, including multiple myeloma, a cancer of the blood-forming bone marrow.
ATMPs - Advanced Therapy Medicinal Products
Somatic cell therapy medicinal products that have been substantially manipulated for use in the body - for example, by propagation in cell culture as in the case of Holoclar and Alofisel - are referred to as ATMPs (advanced therapy medicinal products). The German national regulatory authority for ATMPs is the Paul Ehrlich Institute (PEI), while the European regulatory body is the EMA Committee for Advanced Therapies (CAT). According to the definition given by these regulatory bodies, this category also includes all gene therapy medicines as well as biotechnologically processed tissue products (‘tissue-engineered medicines’) such as skin cells grown in cell culture for transplantation into patients who have suffered severe burns.
Normal skin grafts for burns treatment are not considered ATMPs. Bone marrow transplants with haematopoietic stem cells are also not considered ATMPs unless they have been substantially manipulated (see schematic). Vaccines are also not considered ATMPs, nor are recombinant viral or DNA/RNA vaccines against infectious diseases, such as those widely used in the COVID-19 pandemic. Their mechanism of action is tailored to the pathogen, and human genomes are not altered in the process. However, if a recombinant vaccine is to be used for something completely different than it was initially developed for (namely, for treating cancer instead of for immunisation), then it would have to be classified as an ATMP.
Gene therapeutics
Glybera was the first gene therapy approved in Europe. This was back in 2012 for the treatment of familial (i.e. genetic) lipoprotein lipase (LPL) deficiency. LPL deficiency disrupts the breakdown of fats in the body, resulting in an increase of certain kinds of fats in the blood; severe metabolic disorders and damage to the pancreas and liver then result. Glybera uses an altered adeno-associated virus as a vector or gene shuttle to introduce an intact LPL gene into a patient’s body. Excitement that the approval of Glybera could be a breakthrough in a future medical technology was short-lived. Glybera showed only limited efficacy in the treatment of this extremely rare metabolic hereditary disease, and it was very expensive. In addition, familial LPL deficiency is very rare – affecting only around one in a million individuals in Europe. In the end, there was only one paying patient in Germany outside of the clinical trial that had been performed; the treatment cost almost one million euros. In 2017, Glybera was taken off the market.
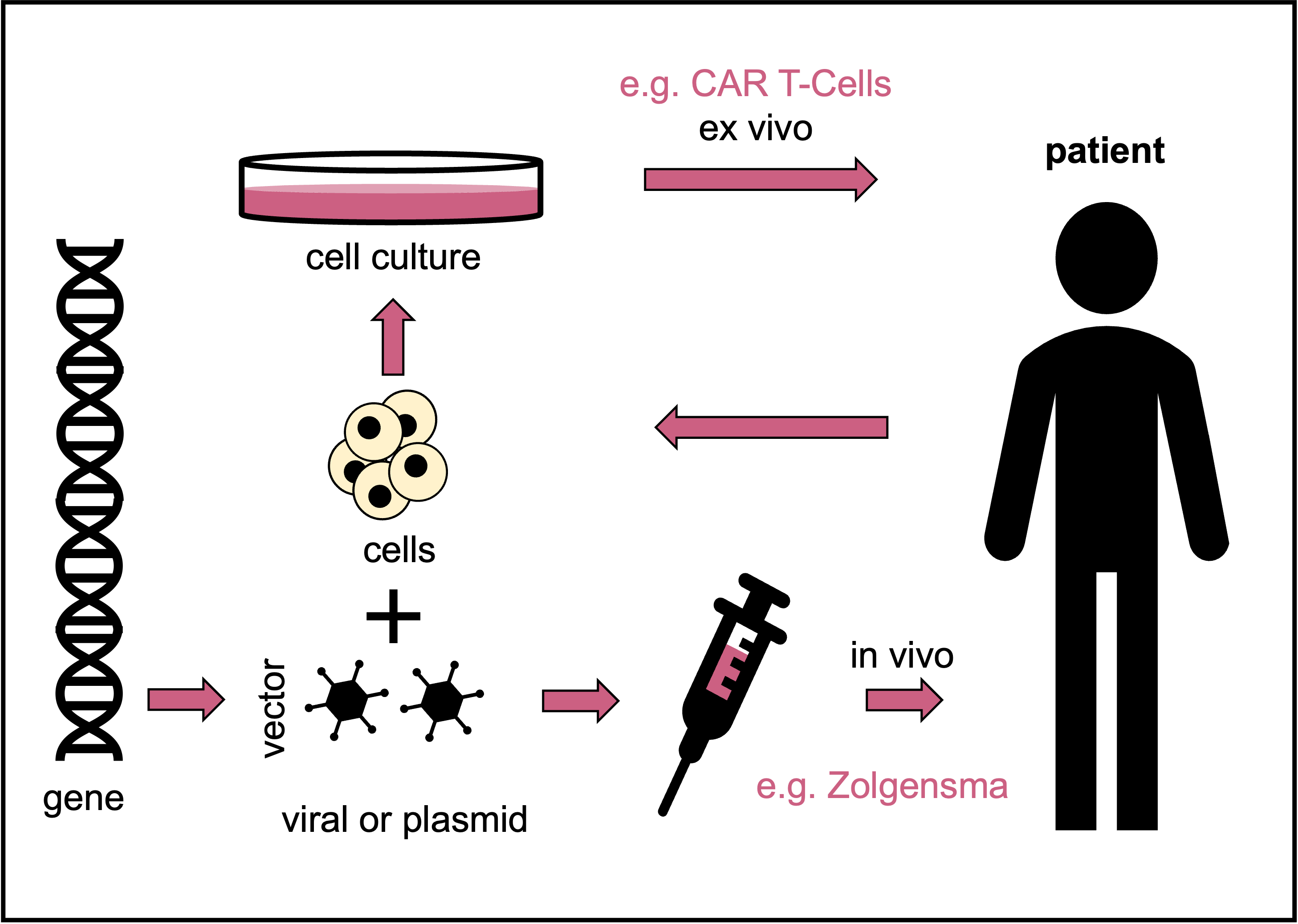 Schematic depicting the terms and their definitions used in tissue, cell and gene therapies and ATMPs. © Dr. Ernst-Dieter Jarasch (adapted from: Karin Hogedoorn & Heather Main 2020, https://atmpsweden.se; graphic implementation: Jana Friedl/BIOPRO Baden-Württemberg)
Schematic depicting the terms and their definitions used in tissue, cell and gene therapies and ATMPs. © Dr. Ernst-Dieter Jarasch (adapted from: Karin Hogedoorn & Heather Main 2020, https://atmpsweden.se; graphic implementation: Jana Friedl/BIOPRO Baden-Württemberg)According to the PEI, ten more gene therapy drugs have since been approved in Europe. However, this does not mean that they are available. Skysona, for example, was withdrawn from the market by the U.S. manufacturer bluebird bio in September 2021, just three months after approval, following unsuccessful cost negotiations with health insurers. This gene therapy is used to treat a very rare hereditary metabolic disease in children by inserting a functional copy of the defective gene into the patients’ blood stem cells using a viral vector; cerebral adrenoleukodystrophy leads to central nervous system damage and often to the early death of those affected.
Bluebird bio also wants to withdraw Zenteglo from the European market: Zynteglo is the first approved gene therapy for children and adolescents with the hereditary disease ß-thalassaemia, but the price tag of close to 1.6 million euros per treatment is not covered by the health insurance companies. In thalassaemias, the red blood pigment haemoglobin cannot be formed properly, resulting in oxygen deficiency in the blood and – with the breakdown of the defective erythrocytes – a pathological accumulation of iron. The health insurance companies argue that there already exists an alternative treatment – namely bone marrow transplants, i.e. the allogeneic transplantation of unmodified blood stem cells, which costs only a fraction of the Zenteglo therapy. Zynteglo is an autologous transplantation of blood stem cells modified ex vivo (i.e. outside the body) into which an intact variant of the defective haemoglobin gene has been introduced with the aid of a viral vector.
However, Prof. Andreas Kulozik of Heidelberg University Hospital, who conducted a successful clinical trial with Zynteglo in the run-up to approval, emphasises that the gene therapy is only indicated for a small group of ß-thalassaemia patients with a precisely defined clinical picture for whom no suitable related stem cell donor is available. About half of all children and adolescents suffering from ß-thalassaemia cannot find such a suitable donor. Bluebird bio says it is now looking for a partner to take over the commercialisation of the Zynteglo and Skysona gene therapies, so there is still hope that they will be available on the European market in the future.
The reasons given for the exorbitant costs of gene therapy are the highly complex treatment process, the costly production of the gene shuttles, the procedure, which is individually tailored to each patient, low patient numbers, complex and long studies and high safety requirements. Zolgensma, an in vivo gene replacement therapy for spinal muscular atrophy, is currently considered the most expensive drug of all time, costing over 2.2 million euros per treatment. This hereditary disease leads to death or severe disabilities in even young children due to muscle atrophy. The cause is a genetic defect that damages the motor neurones in the spinal cord. Zolgensma, now marketed by Swiss pharmaceutical giant Novartis, has been available in Germany since 2020, and 19 children have been treated with Zolgensma at Heidelberg University Hospital alone.
CAR T-cell therapy
Novartis also received European marketing authorisation in August 2018 for its CAR T-cell therapy Kymriah for treating certain blood cancers (B-cell acute lymphoblastic leukaemias and B-cell lymphomas). CAR T-cells are autologous immune cells (T lymphocytes) derived from cancer patients and genetically engineered ex vivo. In the process, they are linked to an artificial receptor for a specific cancer antigen, the chimeric antigen receptor (CAR). Once transplanted back into the patient, the CAR T-cells can find and destroy the cancer cells with the help of this receptor. Despite its name, CAR T-cell therapy is usually classified as gene therapy, but it can also be called immunotherapy with equal justification. [With such novel technologies, definitions often overlap, and it is best to refer to them simply as ATMPs.]
CAR T-cell therapies, although often associated with severe side effects, have become the hottest topic of all ‘advanced therapy medicinal products’; there are hundreds of clinical trials for cancers that could not be treated any other way. Several biopharmaceutical companies have already received approvals for their CAR T-cell therapies – all so far for various haematologic and lymphatic cancers. Recently, however, there have been promising modified approaches for treating solid tumours such as prostate and pancreatic carcinoma or lung cancer with this technology. CAR T-cell therapies are one of the great hopes for the development of the field of oncology and for cancer treatments.